��Ŀ����
����Ŀ��CH4��H2��C�������ʵ���Դ���ʣ�����ȼ�յ��Ȼ�ѧ����ʽΪ��
��CH4(g)��2O2(g)=CO2(g)��2H2O(l) ��H����890.3 kJ��mol��1
��2H2(g)��O2(g)=2H2O(l) ��H����571.6 kJ��mol��1
��C(s)��O2(g)=CO2(g) ��H����393.5 kJ��mol��1
��1������д���һ�ּ���ϸ������������øʹ������O2���ò���������������ϸ��ʹ1 mol��������CO2������Һ̬ˮ���ų�������____________(���������������)890.3 kJ��
��2��������CO2�����ںϳɺϳ���(��Ҫ�ɷ���һ����̼������)��CH4��CO2=2CO��2H2��1 g CH4��ȫ��Ӧ���ͷ�15.46 kJ����������
���ܱ�ʾ�÷�Ӧ�����������仯����____________(����ĸ)��

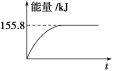
���������ʵ�����Ϊ1 mol��CH4��CO2����ij�����ܱ������У���ϵ�ų�����������ʱ��ı仯��ͼ��ʾ����CH4��ת����Ϊ____________��
��3��C(s)��H2(g)����Ӧ������C(s)��2H2(g)=CH4(g)�ķ�Ӧ����ֱ�Ӳ�������ͨ��������Ӧ�����C(s)��2H2(g)=CH4(g)�ķ�Ӧ�Ȧ�H��____________��
��4��Ŀǰ���������������ʵ��о���ȼ���о����ص㣬���й��������������ʵ��о������п��е���_______________(����ĸ)��
A��Ѱ�����ʴ�����ʹCO2��H2O��Ӧ����CH4��O2�����ų�����
B��Ѱ�����ʴ������ڳ��³�ѹ��ʹCO2�ֽ�����̼��O2
C��Ѱ�����ʴ���������̫����ʹ�����е�CO2�뺣���ɵ�CH4�ϳɺϳ���(CO��H2)
D������̬̼�ϳ�ΪC60����C60��Ϊȼ��
���𰸡���1������2����D��63%��3����74.8kJ��mol��1��4��C
��������
�����������1��������Ӧ�ķ�Ӧ��ֻȡ���ڷ�Ӧ���������Ķ��ٺ�״̬�����м�����أ�����ϸ��ʹ1 mol��������CO2������Һ̬ˮ���ų��������Ե���890.3 kJ��
��2����1g CH4��ȫ��Ӧ���ͷ�15.46 kJ����������1 mol CH4��ȫ��Ӧ�ų�����Ϊ15.46kJ��16=247.36 kJ���÷�ӦΪ���ȷ�Ӧ��1mol�����1molCO2���������ʹ���2molCO��2molH2���������ͣ���ѡD��
�ڸ�����ͼ�ṩ����Ϣ��CH4��ת���ʣ�155.8/247.36 ��100%��63%��
��3�����ݸ�˹���ɣ��ڣ��ۣ��ټ���C(s)��2H2(g)=CH4(g) ��H����571.6 kJ��mol��1��393.5 kJ��mol��1+890.3 kJ��mol��1=��74.8 kJ��mol��1��
��4��A.��֪CH4(g)��2O2(g)=CO2(g)��2H2O(l) ��H����890.3 kJ��mol��1����CO2��H2O��Ӧ����CH4��O2�ķ�Ӧ�����ȷ�Ӧ��A�����B.ʹCO2�ֽ�����̼��O2�ķ�ӦΪ���ȷ�Ӧ�������²��ܷ�����B�����C.����̫����ʹ�����е�CO2�뺣���ɵ�CH4�ϳɺϳ���(CO��H2)�Ǻ����ģ�C����ȷ��D.����̬̼�ϳ�ΪC60����C60��Ϊȼ�ϣ��������ú��㣬D�����ѡC��


