��Ŀ����
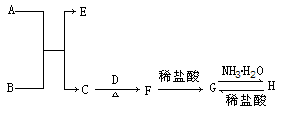
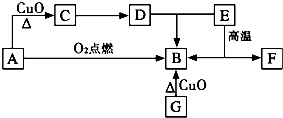 A��������������л���û����������������������ܶ���23��������ȼ��֮һ��C����Һ�ܷ���������Ӧ����Ӧ�����Һ���ữ�ɵ�D��GΪ���ʣ���ԭ������������Ϊ������2����F���������Ӹ�����Ϊ1��1������������Ar������ͬ�ĵ��Ӳ�ṹ��A-F��ת����ϵ��ͼ��?
A��������������л���û����������������������ܶ���23��������ȼ��֮һ��C����Һ�ܷ���������Ӧ����Ӧ�����Һ���ữ�ɵ�D��GΪ���ʣ���ԭ������������Ϊ������2����F���������Ӹ�����Ϊ1��1������������Ar������ͬ�ĵ��Ӳ�ṹ��A-F��ת����ϵ��ͼ��?��1��D��E��Ӧ����B�����ӷ���ʽ��
��2������G�е�Ԫ��λ�ڵ�
��3��I�Ľṹʽ��
��4����I�Ʊ�A��������Ӧ�Ļ�ѧ����ʽ��
��5��G��F�ڸ����·�Ӧ���ɹ���H�Ļ�ѧ��Ӧ����ʽ��
������A��������������л���û����������������������ܶ���23����֪A����Է�������δ46�����A������ȼ��֮һ����֪AΪ�Ҵ���A����������C�����C����Һ�ܷ���������Ӧ����֪CΪ��ȩ����ȩ����Һ�ܷ���������Ӧ����Ӧ�����Һ���ữ�ɵ�D����DΪ���ᣮGΪ���ʣ���ԭ������������Ϊ������2������GΪ̼��̼������ͭ��Ӧ����B���Ҵ���������ȼ������B����BΪ������̼��F���������Ӹ�����Ϊ1��1������������Ar������ͬ�ĵ��Ӳ�ṹ���������ӿ���Ϊ�����ӻ�����ӣ����E���·ֽ�����CO2��F��F���������Ӹ�����Ϊ1��1��FΪ�����ƣ�EΪ̼��ƣ��ݴ˿��ƣ�
����⣺A��������������л���û����������������������ܶ���23����֪A����Է�������δ46�����A������ȼ��֮һ����֪AΪ�Ҵ���A����������C�����C����Һ�ܷ���������Ӧ����֪CΪ��ȩ����ȩ����Һ�ܷ���������Ӧ����Ӧ�����Һ���ữ�ɵ�D����DΪ���ᣮGΪ���ʣ���ԭ������������Ϊ������2������GΪ̼��̼������ͭ��Ӧ����B���Ҵ���������ȼ������B����BΪ������̼��F���������Ӹ�����Ϊ1��1������������Ar������ͬ�ĵ��Ӳ�ṹ���������ӿ���Ϊ�����ӻ�����ӣ����E���·ֽ�����CO2��F��F���������Ӹ�����Ϊ1��1��FΪ�����ƣ�EΪ̼���
��1��DΪ���ᣬEΪ̼��ƣ�D��E��Ӧ�Ļ�ѧ����ʽΪCaCO3+2CH3COOH��Ca2++2CH3COO-+CO2��+H2O��
�ʴ�Ϊ��CaCO3+2CH3COOH��Ca2++2CH3COO-+CO2��+H2O��
��2��̼λ�ڵڶ����ڵ������壬�ʴ�Ϊ��������A��
��3��IΪ��Ȳ����ṹ��ʽH-C��C-H���ʴ�Ϊ��H-C��C-H��
��4����Ȳ�����Ҵ���������ˮ�����ӳɷ�Ӧ������ȩ���ױ���������Ӧ�������Ҵ�����Ӧ�ķ���ʽΪC2H2+H2O
CH3CHO��CH3CHO+H2
CH3CH2OH��
�ʴ�Ϊ��C2H2+H2O
CH3CHO��CH3CHO+H2
CH3CH2OH��
��5��G��F�ڸ����·�Ӧ���ɹ���H�Ļ�ѧ��Ӧ����ʽΪCaO+3C
CaC2+CO�����ʴ�Ϊ��CaO+3C
CaC2+CO����
��1��DΪ���ᣬEΪ̼��ƣ�D��E��Ӧ�Ļ�ѧ����ʽΪCaCO3+2CH3COOH��Ca2++2CH3COO-+CO2��+H2O��
�ʴ�Ϊ��CaCO3+2CH3COOH��Ca2++2CH3COO-+CO2��+H2O��
��2��̼λ�ڵڶ����ڵ������壬�ʴ�Ϊ��������A��
��3��IΪ��Ȳ����ṹ��ʽH-C��C-H���ʴ�Ϊ��H-C��C-H��
��4����Ȳ�����Ҵ���������ˮ�����ӳɷ�Ӧ������ȩ���ױ���������Ӧ�������Ҵ�����Ӧ�ķ���ʽΪC2H2+H2O
| ���� |
| ���ȡ���ѹ |
| ���� |
| ���� |
�ʴ�Ϊ��C2H2+H2O
| ���� |
| ���ȡ���ѹ |
| ���� |
| ���� |
��5��G��F�ڸ����·�Ӧ���ɹ���H�Ļ�ѧ��Ӧ����ʽΪCaO+3C
| ||
| ||
����������Ϊ�л��ƶ��⣬��Ҫ�����˴���ȩ����������ʣ�����Ȳ���Ʒ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ