��Ŀ����
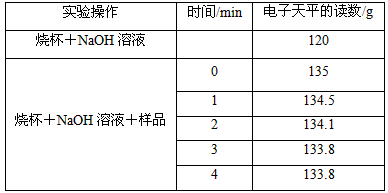
 ��ͼ�DzⶨNaOH����Na2CO3���ʣ����ȵ�ʵ��װ�ã�����Ʒ���ڹ����������У���ͬʱ�������ƽ�Ķ������±���ʾ������CO2ȫ���ݳ�����
��ͼ�DzⶨNaOH����Na2CO3���ʣ����ȵ�ʵ��װ�ã�����Ʒ���ڹ����������У���ͬʱ�������ƽ�Ķ������±���ʾ������CO2ȫ���ݳ�����
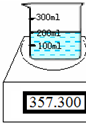
| ʵ����� | ʱ��/s | ������ƽ����/g |
| �ձ�+���� | 357.300 | |
�ձ�+����+��Ʒ | 0 30 60 90 120 | 370.600 370.125 369.750 368.400 368.400 |
��2�����Լ�����Һ�������ɵ����ʵ����ʵ���Ũ�ȣ�
�⣨1���ɱ������ݿ�֪���ձ��������������Ϊ357.300g��������Ʒ��0s��������Ϊ370.600g������Ʒ������Ϊ370.600g-357.300g=13.3g����90s��120s����������֪����Ӧ��ȫ������������370.600g-368.400g=2.2g����������Ϊ���ɶ�����̼���������ʶ�����̼�����ʵ���Ϊ =0.05mol����̼ԭ���غ��֪̼���Ƶ����ʵ���Ϊ0.05mol����̼���Ƶ�����Ϊ0.05mol��106g/mol=5.3g������Ʒ���������Ƶ�����Ϊ13.3g-5.3g=8g��
=0.05mol����̼ԭ���غ��֪̼���Ƶ����ʵ���Ϊ0.05mol����̼���Ƶ�����Ϊ0.05mol��106g/mol=5.3g������Ʒ���������Ƶ�����Ϊ13.3g-5.3g=8g��
����Ʒ���������Ƶ�����Ϊ8g��
��2��8g�������Ƶ����ʵ���Ϊ =0.2mol�������ɵ�����ΪNaCl��������ԭ���غ��֪��Һ��n��NaCl��=n��NaOH��+2n��Na2CO3��=0.2mol+0.05mol��2=0.3mol����Һ�����Ϊ200mL�����Ȼ��Ƶ�Ũ��Ϊ
=0.2mol�������ɵ�����ΪNaCl��������ԭ���غ��֪��Һ��n��NaCl��=n��NaOH��+2n��Na2CO3��=0.2mol+0.05mol��2=0.3mol����Һ�����Ϊ200mL�����Ȼ��Ƶ�Ũ��Ϊ =1.5mol/L��
=1.5mol/L��
����Һ�������ɵ�NaCl�����ʵ���Ũ��Ϊ1.5mol/L��
��������1���ɱ������ݿ�֪���ձ��������������Ϊ357.300g��������Ʒ��0s��������Ϊ370.600g������Ʒ������Ϊ370.600g-357.300g=13.3g����90s��120s����������֪����Ӧ��ȫ������������370.600g-368.400g=2.2g����������Ϊ���ɶ�����̼������������n=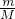 ���������̼�����ʵ�������̼ԭ���غ����̼���Ƶ����ʵ���������m=nM����̼���Ƶ�����������������Ʒ���������Ƶ�������
���������̼�����ʵ�������̼ԭ���غ����̼���Ƶ����ʵ���������m=nM����̼���Ƶ�����������������Ʒ���������Ƶ�������
��2�������ɵ�����ΪNaCl��������ԭ���غ��֪��Һ��n��NaCl��=n��NaOH��+2n��Na2CO3�����ٸ���c=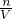 ���������ɵ�NaCl�����ʵ���Ũ�ȣ�
���������ɵ�NaCl�����ʵ���Ũ�ȣ�
����������������йؼ��㣬�Ѷ��еȣ���1����ע���غ�˼������ã�
 =0.05mol����̼ԭ���غ��֪̼���Ƶ����ʵ���Ϊ0.05mol����̼���Ƶ�����Ϊ0.05mol��106g/mol=5.3g������Ʒ���������Ƶ�����Ϊ13.3g-5.3g=8g��
=0.05mol����̼ԭ���غ��֪̼���Ƶ����ʵ���Ϊ0.05mol����̼���Ƶ�����Ϊ0.05mol��106g/mol=5.3g������Ʒ���������Ƶ�����Ϊ13.3g-5.3g=8g������Ʒ���������Ƶ�����Ϊ8g��
��2��8g�������Ƶ����ʵ���Ϊ
 =0.2mol�������ɵ�����ΪNaCl��������ԭ���غ��֪��Һ��n��NaCl��=n��NaOH��+2n��Na2CO3��=0.2mol+0.05mol��2=0.3mol����Һ�����Ϊ200mL�����Ȼ��Ƶ�Ũ��Ϊ
=0.2mol�������ɵ�����ΪNaCl��������ԭ���غ��֪��Һ��n��NaCl��=n��NaOH��+2n��Na2CO3��=0.2mol+0.05mol��2=0.3mol����Һ�����Ϊ200mL�����Ȼ��Ƶ�Ũ��Ϊ =1.5mol/L��
=1.5mol/L������Һ�������ɵ�NaCl�����ʵ���Ũ��Ϊ1.5mol/L��
��������1���ɱ������ݿ�֪���ձ��������������Ϊ357.300g��������Ʒ��0s��������Ϊ370.600g������Ʒ������Ϊ370.600g-357.300g=13.3g����90s��120s����������֪����Ӧ��ȫ������������370.600g-368.400g=2.2g����������Ϊ���ɶ�����̼������������n=
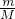 ���������̼�����ʵ�������̼ԭ���غ����̼���Ƶ����ʵ���������m=nM����̼���Ƶ�����������������Ʒ���������Ƶ�������
���������̼�����ʵ�������̼ԭ���غ����̼���Ƶ����ʵ���������m=nM����̼���Ƶ�����������������Ʒ���������Ƶ���������2�������ɵ�����ΪNaCl��������ԭ���غ��֪��Һ��n��NaCl��=n��NaOH��+2n��Na2CO3�����ٸ���c=
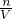 ���������ɵ�NaCl�����ʵ���Ũ�ȣ�
���������ɵ�NaCl�����ʵ���Ũ�ȣ�����������������йؼ��㣬�Ѷ��еȣ���1����ע���غ�˼������ã�

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
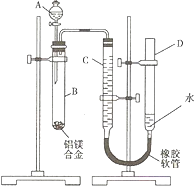 ��2008?���죩ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ��������
��2008?���죩ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ�������� �������ʵ���Ũ��Ϊa mol?L-1�ı�NaOH��Һȥ�ζ�V mL��������ʵ���Ũ�ȣ�����д���пհף�
�������ʵ���Ũ��Ϊa mol?L-1�ı�NaOH��Һȥ�ζ�V mL��������ʵ���Ũ�ȣ�����д���пհף�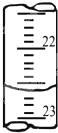 ijѧ����0.2000mol?L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ���¼�����
ijѧ����0.2000mol?L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ���¼�����