ΧβΡΩΡΎ»ί
»γΆΦ÷–Θ§AΓΔBΓΔCΓΔDΓΔE «ΒΞ÷ Θ§GΓΔHΓΔIΓΔF «BΓΔCΓΔDΓΔEΖ÷±πΚΆA–Έ≥…ΒΡΕΰ‘ΣΜ·ΚœΈοΓΘ“―÷ΣΘΚ
ΔΌΖ¥”ΠC+G B+HΡήΖ≈≥ω¥σΝΩΒΡ»»Θ§ΗΟΖ¥”Π‘χ”Π”Ο”ΎΧζΙλΒΡΚΗΫ”ΘΜ
B+HΡήΖ≈≥ω¥σΝΩΒΡ»»Θ§ΗΟΖ¥”Π‘χ”Π”Ο”ΎΧζΙλΒΡΚΗΫ”ΘΜ
ΔΎI «“Μ÷÷≥ΘΦϊΒΡΈ¬ “ΤχΧεΘ§ΥϋΚΆEΩ…“‘ΖΔ…ζΖ¥”ΠΘΚ2E+I 2F+DΘ§F÷–E‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΈΣ60%ΓΘ
2F+DΘ§F÷–E‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΈΣ60%ΓΘ

ΜΊ¥πΈ ΧβΘΚ
Θ®1Θ©ΔΌ÷–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ________________________________________ΘΜ
Θ®2Θ©1.6 g G»ή”Ύ―ΈΥαΘ§ΒΟΒΫΒΡ»ή“Κ”κΆ≠ΖέΆξ»ΪΖ¥”ΠΘ§ΦΤΥψ÷Ν…Ό–ηΆ≠ΖέΒΡ÷ ΝΩΘ®–¥≥ωάκΉ”ΖΫ≥Χ ΫΚΆΦΤΥψΙΐ≥ΧΘ©ΘΜ__________________________________________ΓΘ
Θ®3Θ©C”κΙΐΝΩNaOH»ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚ___________________________Θ§Ζ¥”ΠΚσ»ή“Κ”κΙΐΝΩΜ·ΚœΈοIΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚ_________________________ΘΜ
Θ®4Θ©E‘ΎI÷–»Φ…’Ιέ≤λΒΫΒΡœ÷œσ «ΘΚ____________________________________ΓΘ
Θ®1Θ©
Θ®2Θ©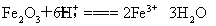

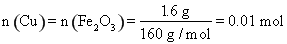 Θ§÷Ν…Ό–ηΆ≠ΖέΒΡ÷ ΝΩΈΣ0.01 molΓΝ64 g/mol=0.64 g
Θ§÷Ν…Ό–ηΆ≠ΖέΒΡ÷ ΝΩΈΣ0.01 molΓΝ64 g/mol=0.64 g
Θ®3Θ©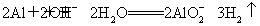

Θ®4Θ©ΟΨΧθΨγΝ“»Φ…’Θ§…ζ≥…ΑΉ…ΪΖέΡ©Θ§Ζ¥”ΠΤςΡΎ±ΎΗΫΉ≈ΚΎ…ΪΙΧΧε
ΓΨΫβΈωΓΩ±ΨΧβ÷ς“ΣΩΦ≤ιMgΓΔAlΓΔFeΦΑΤδΜ·ΚœΈοΒΡ–‘÷ ΦΑ”κΤδ”–ΙΊΒΡΜ·―ßΖΫ≥Χ ΫΒΡΦΤΥψΓΘΫβΧβ ±ΉΔ“β―Α’“ΆΜΤΤΩΎΘ§»γ¬Ν»»Ζ¥”Π”Π”Ο”ΎΧζΙλΒΡΚΗΫ”ΓΔCO2 «Ήν≥ΘΦϊΒΡΈ¬ “ΤχΧεΒ»ΓΘ
Θ®1Θ©¬Ν»»Ζ¥”Π”Π”Ο”ΎΧζΙλΒΡΚΗΫ”Θ§”…ΔΌΩ…÷ΣCΈΣAlΓΔGΈΣFe2O3ΓΔBΈΣFeΓΔHΈΣAl2O3Θ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ2AlΘΪFe2O3 2FeΘΪAl2O3ΓΘCO2 «Ήν≥ΘΦϊΒΡΈ¬ “ΤχΧεΘ§”…ΔΎΩ…÷ΣEΈΣMgΓΔIΈΣCO2ΓΔFΈΣMgOΓΔDΈΣCΓΘΉέ…œΩ…÷ΣAΈΣO2ΓΘ
2FeΘΪAl2O3ΓΘCO2 «Ήν≥ΘΦϊΒΡΈ¬ “ΤχΧεΘ§”…ΔΎΩ…÷ΣEΈΣMgΓΔIΈΣCO2ΓΔFΈΣMgOΓΔDΈΣCΓΘΉέ…œΩ…÷ΣAΈΣO2ΓΘ
Θ®2Θ©Fe2O3”κ―ΈΥαΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣFe2O3ΘΪ6H+=2Fe3+ΘΪ3H2OΘ§FeCl3”κΆ≠ΖέΆξ»ΪΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ2Fe3+ΘΪCu=2Fe2+ΘΪCu2+Θ§Ω…÷ΣnΘ®CuΘ©=nΘ®Fe2O3Θ©=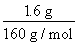 =0.01 mol,–ηΆ≠ΖέΒΡ÷ ΝΩΈΣ0.01 molΓΝ64 g/mol=0.64 gΓΘ
=0.01 mol,–ηΆ≠ΖέΒΡ÷ ΝΩΈΣ0.01 molΓΝ64 g/mol=0.64 gΓΘ
Θ®3Θ©Al”κΙΐΝΩNaOH»ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚ2AlΘΪ2OH-ΘΪ2H2O= ΘΪ
ΘΪ
3H2ΓϋΘ§Ζ¥”ΠΚσ»ή“ΚΈΣNaAlO2Θ§”κΙΐΝΩCO2Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚ ΓΘ
ΓΘ
Θ®4Θ©Mg‘ΎCO2÷–»Φ…’ΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ2Mg+CO2 2MgOΘΪCΘ§Ιέ≤λΒΫΒΡœ÷œσ «ΟΨΧθΨγΝ“»Φ…’Θ§…ζ≥…ΑΉ…ΪΖέΡ©Θ§Ζ¥”ΠΤςΡΎ±ΎΗΫΉ≈ΚΎ…ΪΙΧΧεΓΘ
2MgOΘΪCΘ§Ιέ≤λΒΫΒΡœ÷œσ «ΟΨΧθΨγΝ“»Φ…’Θ§…ζ≥…ΑΉ…ΪΖέΡ©Θ§Ζ¥”ΠΤςΡΎ±ΎΗΫΉ≈ΚΎ…ΪΙΧΧεΓΘ



