��Ŀ����
Q��R��W��X��Y��ZΪ6�ֶ�����Ԫ�أ���ԭ��������������R��W��Xͬ���ڣ�X��Zͬ���壮Q��R������ԭ�Ӹ�����4��1�γɻ�����ף��Ҽ����к���10�����ӣ�Q��W�γɻ������ң��ҿ���Z������������ˮ���ﰴ�����ʵ���֮��2��1��Ӧ�γ��α���Y��ԭ��������R��Zԭ�������͵�һ�룮
��1��XԪ����Ԫ�����ڱ��е�λ���� ��
��2���ĵ���ʽ�� ���ҵĽṹʽ ��
��3��X��Y�γɵļȺ����Լ��ֺ��Ǽ��Լ��Ĺ��ۻ�����Ļ�ѧʽ�� ��
��4������������Һ�м������ŨBa��OH��2��Һ�����ȣ���Ӧ�����ӷ���ʽ�� ��
��5���Һ�Ԫ��X�ĵ��ʷ�Ӧ��ij���������Ļ�����д���˷�Ӧ�Ļ�ѧ����ʽ�� ��
��6�����������YԪ������������ˮ�����ϡ��Һ�У�ˮ�ĵ���̶Ȳ�ͬ��ԭ���� ��
��1��XԪ����Ԫ�����ڱ��е�λ����
��2���ĵ���ʽ��
��3��X��Y�γɵļȺ����Լ��ֺ��Ǽ��Լ��Ĺ��ۻ�����Ļ�ѧʽ��
��4������������Һ�м������ŨBa��OH��2��Һ�����ȣ���Ӧ�����ӷ���ʽ��
��5���Һ�Ԫ��X�ĵ��ʷ�Ӧ��ij���������Ļ�����д���˷�Ӧ�Ļ�ѧ����ʽ��
��6�����������YԪ������������ˮ�����ϡ��Һ�У�ˮ�ĵ���̶Ȳ�ͬ��ԭ����
������Q��R��W��X��Y��ZΪ6�ֶ�����Ԫ�أ���ԭ��������������Q��R������ԭ�Ӹ�����4��1�γɻ�����ף��Ҽ����к���10�����ӣ����ΪCH4��QΪHԪ�ء�RΪCԪ�أ�Q��W�γɻ�������Ϊ�⻯��ҿ���Z������������ˮ���ﰴ�����ʵ���֮��2��1��Ӧ�γ��α�������ΪNH3����Ϊ��Σ�Z������������ˮ����Ϊ��Ԫ�ᣬ��ӦΪ��NH4��2SO4����WΪNԪ�أ�ZΪSԪ�أ�X��Zͬ���壬XΪOԪ�أ�Y��ԭ��������R��Zԭ�������͵�һ�룬��Yԭ������Ϊ
����6+16��=11����YΪNaԪ�أ��ݴ˽��
| 1 |
| 2 |
����⣺Q��R��W��X��Y��ZΪ6�ֶ�����Ԫ�أ���ԭ��������������Q��R������ԭ�Ӹ�����4��1�γɻ�����ף��Ҽ����к���10�����ӣ����ΪCH4��QΪHԪ�ء�RΪCԪ�أ�Q��W�γɻ�������Ϊ�⻯��ҿ���Z������������ˮ���ﰴ�����ʵ���֮��2��1��Ӧ�γ��α�������ΪNH3����Ϊ��Σ�Z������������ˮ����Ϊ��Ԫ�ᣬ��ӦΪ��NH4��2SO4����WΪNԪ�أ�ZΪSԪ�أ�X��Zͬ���壬XΪOԪ�أ�Y��ԭ��������R��Zԭ�������͵�һ�룬��Yԭ������Ϊ
����6+16��=11����YΪNaԪ�أ�
��1��XΪOԪ�أ�����Ԫ�����ڱ��еڶ����ڵڢ�A�壬
�ʴ�Ϊ���ڶ����ڵڢ�A�壻
��2����ΪCH4�������ʽΪ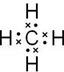 ����ΪNH3����ṹʽΪ
����ΪNH3����ṹʽΪ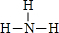 ��
��
�ʴ�Ϊ�� ��
��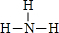 ��
��
��3��O��Na�γɵļȺ����Լ��ֺ��Ǽ��Լ��Ĺ��ۻ�����Ļ�ѧʽ��Na2O2��
�ʴ�Ϊ��Na2O2��
��4����������NH4��2SO4��Һ�м������ŨBa��OH��2��Һ�����ȣ���Ӧ�����ӷ���ʽΪ��2NH4++SO42-+Ba2++2OH-
BaSO4��+2H2O+2NH3����
�ʴ�Ϊ��2NH4++SO42-+Ba2++2OH-
BaSO4��+2H2O+2NH3����
��5��NH3��������Ӧ�ǹ���������Ļ������÷�Ӧ�Ļ�ѧ����ʽΪ��4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2
4NO+6H2O��
��6�������NH4��2SO4��YԪ������������ˮ����NaOH��ϡ��Һ�У�ˮ�ĵ���̶Ȳ�ͬ��ԭ���ǣ�����NH4��2SO4ˮ��ٽ�ˮ�ĵ��룬��NaOH����ˮ�ĵ��룬
�ʴ�Ϊ����NH4��2SO4ˮ��ٽ�ˮ�ĵ��룬��NaOH����ˮ�ĵ��룮
| 1 |
| 2 |
��1��XΪOԪ�أ�����Ԫ�����ڱ��еڶ����ڵڢ�A�壬
�ʴ�Ϊ���ڶ����ڵڢ�A�壻
��2����ΪCH4�������ʽΪ
 ����ΪNH3����ṹʽΪ
����ΪNH3����ṹʽΪ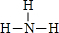 ��
���ʴ�Ϊ��
 ��
��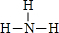 ��
����3��O��Na�γɵļȺ����Լ��ֺ��Ǽ��Լ��Ĺ��ۻ�����Ļ�ѧʽ��Na2O2��
�ʴ�Ϊ��Na2O2��
��4����������NH4��2SO4��Һ�м������ŨBa��OH��2��Һ�����ȣ���Ӧ�����ӷ���ʽΪ��2NH4++SO42-+Ba2++2OH-
| ||
�ʴ�Ϊ��2NH4++SO42-+Ba2++2OH-
| ||
��5��NH3��������Ӧ�ǹ���������Ļ������÷�Ӧ�Ļ�ѧ����ʽΪ��4NH3+5O2
| ||
| �� |
�ʴ�Ϊ��4NH3+5O2
| ||
| �� |
��6�������NH4��2SO4��YԪ������������ˮ����NaOH��ϡ��Һ�У�ˮ�ĵ���̶Ȳ�ͬ��ԭ���ǣ�����NH4��2SO4ˮ��ٽ�ˮ�ĵ��룬��NaOH����ˮ�ĵ��룬
�ʴ�Ϊ����NH4��2SO4ˮ��ٽ�ˮ�ĵ��룬��NaOH����ˮ�ĵ��룮
���������⿼��λ�ýṹ���ʹ�ϵӦ�ã���Ŀ�Ѷ��еȣ���ȷ�ƶ�Ԫ���ǽ���Ĺؼ���ע��Ի���֪ʶ�Ļ������գ�

��ϰ��ϵ�д�
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ