��Ŀ����
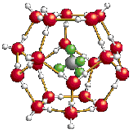 �ҹ�����ظ�ԭ���ִ�����ȼ������ȼ���ľ���ṹģ����ͼ��
�ҹ�����ظ�ԭ���ִ�����ȼ������ȼ���ľ���ṹģ����ͼ����1��C��N��O����Ԫ�ص�һ�������ɴ�С������˳����
��2������ȼ���������ڲ����ڵ���������
��3�������йء���ȼ������˵���У�����ȷ����
A������ȼ�����Ĵ���˵������������ˮ
B������ȼ�������ڷ��Ӿ��壬�۵�ϵ�
C����ͬ�����£�����ȼ�������ܶȱȱ��Ĵ�
D������ȼ����ֻ���ڵ��¡���ѹ�������ȶ�����
��4��������ƽ��ÿ46��ˮ���ӹ���8������ÿ����������1��CH4���ӻ�1��H2O���ӣ�ÿ8��������6������CH4���ӣ�2�������H2O���ӣ���ȼ��������Ȼ��ˮ�����ƽ����ɿɱ�ʾΪ
��������1��ͬһ�����У�Ԫ�صĵ�һ����������ԭ����ȵ���������������ƣ�����IIA����VA��Ԫ�ص�һ�����ܴ�������Ԫ�أ����ݼ۲���ӶԻ�������ȷ��H2O������Oԭ�ӵ��ӻ���ʽ��
��2������ȼ���������ڲ�ԭ��֮����ڻ�ѧ����ˮ������Oԭ�Ӻ�����ˮ�����е�Hԭ���γ���������Ӽ���ڷ��»�����
��3��A�����鲻������ˮ��
B�����Ӿ�����۷е�ϵͣ�
C������֮�䲻���γ���������з��Ӽ������������±����ܶȴ��ڿ�ȼ����
D������ȼ�����ڽϸ��¶Ȼ�ϵ�ѹǿ�£��þ����ױ��ƻ���
��4�����þ�̯��ȷ����ѧʽ��
��2������ȼ���������ڲ�ԭ��֮����ڻ�ѧ����ˮ������Oԭ�Ӻ�����ˮ�����е�Hԭ���γ���������Ӽ���ڷ��»�����
��3��A�����鲻������ˮ��
B�����Ӿ�����۷е�ϵͣ�
C������֮�䲻���γ���������з��Ӽ������������±����ܶȴ��ڿ�ȼ����
D������ȼ�����ڽϸ��¶Ȼ�ϵ�ѹǿ�£��þ����ױ��ƻ���
��4�����þ�̯��ȷ����ѧʽ��
����⣺��1��ͬһ�����У�Ԫ�صĵ�һ����������ԭ����ȵ���������������ƣ�����IIA����VA��Ԫ�ص�һ�����ܴ�������Ԫ�أ�����C��N��O����Ԫ�صĵ�һ�����ܴ�С˳����N��O��C��
H2O������Oԭ�Ӽ۲���ӶԸ���=2+
��6-2��1��=4�Һ���2���µ��Ӷԣ�����Oԭ�Ӳ���sp3�ӻ����ʴ�Ϊ��N��O��C��sp3��
��2������ȼ���������ڲ�ԭ��֮����ڻ�ѧ����ˮ������Oԭ�Ӻ�����ˮ�����е�Hԭ���γ���������Ӽ���ڷ��»����������ڿ�ȼ���������ڲ����ڵ�����������������»��������ۼ����ʴ�Ϊ����������»��������ۼ���
��3��A�����鲻������ˮ������ȼ�����Ĵ��ڲ���˵������������ˮ��˵������CH4?8H2O����A����
B������ȼ�����Ĺ������Ƿ��ӣ����ڷ��Ӿ��壬�۵�ϵͣ���B��ȷ��
C������ȼ�����д���������������д��ڴ������������Ĵ��ڵ��·���֮������С��������ͬ�����£�����ȼ�������ܶȱȱ���С����C����
D������ȼ�������ڷ��Ӿ��壬�Ҽ����������壬�¶ȡ�ѹǿӰ������Ĵ��ڣ�����ֻ���ڵ��¡���ѹ�������ȶ����ڣ���D��ȷ��
��ѡAC��
��4��ƽ��ÿ46��ˮ���ӹ���8������������ÿ8��������6��������CH4���ӣ�����2����2�������H2O��������䣻
��Ȼ��ˮ����Ĺ����к�6��CH4���ӡ�46+2=48��H2O���ӣ���CH4������H2O������������=6��48=1��8��
���ж���Ȼ��ˮ�����ƽ����ɿɱ�ʾΪCH4?8H2O��
�ʴ�Ϊ��CH4?8H2O��
H2O������Oԭ�Ӽ۲���ӶԸ���=2+
| 1 |
| 2 |
��2������ȼ���������ڲ�ԭ��֮����ڻ�ѧ����ˮ������Oԭ�Ӻ�����ˮ�����е�Hԭ���γ���������Ӽ���ڷ��»����������ڿ�ȼ���������ڲ����ڵ�����������������»��������ۼ����ʴ�Ϊ����������»��������ۼ���
��3��A�����鲻������ˮ������ȼ�����Ĵ��ڲ���˵������������ˮ��˵������CH4?8H2O����A����
B������ȼ�����Ĺ������Ƿ��ӣ����ڷ��Ӿ��壬�۵�ϵͣ���B��ȷ��
C������ȼ�����д���������������д��ڴ������������Ĵ��ڵ��·���֮������С��������ͬ�����£�����ȼ�������ܶȱȱ���С����C����
D������ȼ�������ڷ��Ӿ��壬�Ҽ����������壬�¶ȡ�ѹǿӰ������Ĵ��ڣ�����ֻ���ڵ��¡���ѹ�������ȶ����ڣ���D��ȷ��
��ѡAC��
��4��ƽ��ÿ46��ˮ���ӹ���8������������ÿ8��������6��������CH4���ӣ�����2����2�������H2O��������䣻
��Ȼ��ˮ����Ĺ����к�6��CH4���ӡ�46+2=48��H2O���ӣ���CH4������H2O������������=6��48=1��8��
���ж���Ȼ��ˮ�����ƽ����ɿɱ�ʾΪCH4?8H2O��
�ʴ�Ϊ��CH4?8H2O��
���������⿼�������ʽṹ�����ʣ��漰��һ�����ܴ�С���жϡ�ԭ���ӻ���ʽ���жϡ������ļ����֪ʶ�㣬����Ԫ�������ɡ��۲���ӶԻ������ۡ���̯����֪ʶ�������������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
