��Ŀ����
����Ŀ����������һ����Ч����ȫ�Ĺ��崢����ϣ�������Ľṹ���������ƣ���ͼ��
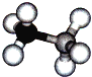
��1��������ľ�������Ϊ__��
��2��������Ƚϣ��縺�Դ��Ԫ��Ϊ__����Ԫ�ط��ţ���
��3������������е�ԭ�ӡ���ԭ�ӵ��ӻ�������ͷֱ�Ϊ__��__��
��4��������__����ܡ����ܡ�������ˮ����ԭ����__��
��5����NaBH4Ϊ��Դ��ij�����Ϊ��Դ�������Ʊ������飮Ϊ�ⶨ�������Ľṹ��ȡ2.32g������������ʵ�飺����������������Ԫ�أ������Ԫ�ص�����Ϊ0.59g���ڼ��������¼�������NH3���õζ�����������ʵ���Ϊ0.06mol���μӹ�����������Һ���а�ɫ�������ɣ����ȣ�����û�����ӣ���Ħ�������������������Ԫ������Ϊ0.71g��
��������������ӵĻ�̬�����Ų�ʽΪ__���������Ļ�ѧʽΪ ��
���𰸡����Ӿ��� N sp3��sp3�� �� �������ˮ֮������γ���� 1s22s22p63s23p63d8 [Ni��NH3��6]Cl2
��������
��1���������ʵĽṹ��ȷ�������ķ��Ӿ������ͣ�
��2���õ�������ǿ��ԭ�ӵ縺�Դ�
��3�����ݷ��ӵĿռ�ṹ�Լ��ɼ�Ԫ��ȷ���ӻ����ͣ�
��4���ܺ�ˮ���Ӽ��γ����������һ����������ˮ�ģ�
��5������ԭ�ӵĻ�̬�����Ų�ʽ����д���ش𣬸���Ԫ���غ����������ʵķ���ʽ��
��1��������ķ��ӽṹ���������ƣ���������ṹ�ķ��Ӿ��壬�ʴ�Ϊ�����Ӿ��壻
��2�����ĵõ�������ǿ���𣬵�����Ƚϣ��縺�Դ��Ԫ��ΪN���ʴ�Ϊ��N��
��3������������е�ԭ�ӡ���ԭ�������������ӣ����ǵ��ӻ�������ͷֱ�Ϊsp3��sp3���ʴ�Ϊ��sp3��sp3��
��4���������ܺ�ˮ���Ӽ��γ����������������ˮ���ʴ�Ϊ���ܣ��������ˮ֮������γ������
��5����Niԭ�ӵ�ԭ��������28�������ӵĻ�̬�����Ų�ʽΪ1s22s22p63s23p63d8���ʴ�Ϊ��1s22s22p63s23p63d8��
������������������Ԫ�أ������Ԫ�ص�����Ϊ0.59g��0.01mol���ڼ��������¼�������NH3���õζ�����������ʵ���Ϊ0.06mol����NԪ�����ʵ�����0.06mol����Ħ�������������������Ԫ������Ϊ0.71g����ClԪ�ص����ʵ�����0.01mol�����������غ㣬HԪ��������0.18g�����Է�����Ni��N��Cl��H��ԭ�Ӹ���֮����1��6��2��18�������������ص㣬�ó���ѧʽΪ��[Ni��NH3��6]Cl2���ʴ�Ϊ��[Ni��NH3��6]Cl2��








