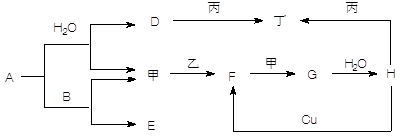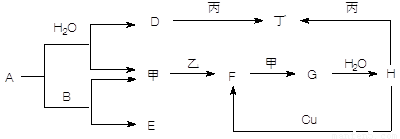��Ŀ����
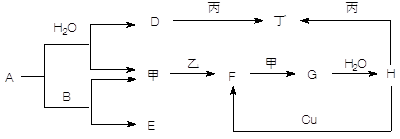
����5�֣���ͼת����ϵ��A��HΪ��ѧ����������ס��ҡ�������Ϊ�������ʣ����мס�������Ϊ���塣��֪�����ҡ�������AΪ��ɫ����ɫ�����塣�Ҿ����������Ӧ������ˮ���ϣ��ǹ�ҵ����H�ķ�Ӧ���̡�B��F�������嶼��ʹ����ʯ��ˮ����ǡ������ַ�Ӧ��������ȥ��
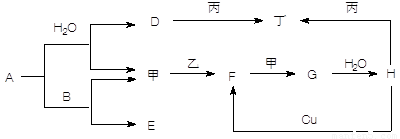
(1��д�����з�Ӧ�Ļ�ѧ����ʽ��
A��B ____________________________________________________
Cu��H ___________________________________________________
F���____________________________________________________
(2��д������D��Һ��Ӧ�����ӷ���ʽ _______________________________________
(3������ʱH��Ũ��Һ������ʲ����ܿ������Եķ�Ӧ����ԭ����_________ ____ ��
(1) 2Na2O2��2CO2 ===2Na2CO3��O2 Cu��2H2SO4��Ũ��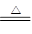 CuSO4��SO2 ����2H2O
CuSO4��SO2 ����2H2O
2SO2+O2 2SO3 (2��2Al��2OH����2H2O��2AlO2����3H2��
2SO3 (2��2Al��2OH����2H2O��2AlO2����3H2��
(3������Ũ��������γ����ܵ�����Ĥ����ֹ�ڲ������ķ�Ӧ��������Ũ�����жۻ���
��������������AΪ��ɫ����ɫ�����壬˵��A�ǹ������ơ���B��F�������嶼��ʹ����ʯ��ˮ����ǣ�����B��CO2��F��SO2����E��̼���ƣ�����������������G����ҵ��¸��H�����ᡣD���������ƣ��������������������

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�