��Ŀ����
(5��) �����£���Ũ�Ⱦ�Ϊ0.5mol/L��������Һ����Na2CO3��NaHCO3��HCl ��NH3?H2O
��1��������Һ�У��ɷ���ˮ����� ������ţ���
��2������Һ���м��������Ȼ�粒��壬��ʱc(NH4+)/c(OH��)��ֵ ��������С�䣩��
��3�������ۺܵ͢���Һ��Ϻ���Һǡ�ó����ԣ�����ǰ�۵���� �ܵ�����������������������������ʱ��Һ������Ũ���ɴ�С��˳���� ��
��4��ȡ10mL��Һ�ۣ���ˮϡ�͵�500mL,�����Һ����ˮ�������c(H+ )= ��
)= ��
��1���٢� (©ѡ����ѡ���÷�)
��2������
��3���� c(NH4+)=c(Cl-)��c(H+)=c(OH-)
��4�� 10-12 mol/L
����

��ϰ��ϵ�д�
�����Ŀ
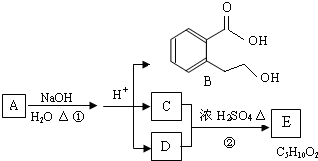

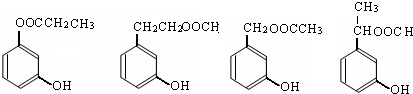 д������֮һ����
д������֮һ����
