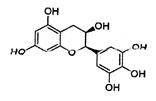
��Ŀ����
Īɳ������һ����ʹҩ�����ĺϳ�·�����£�
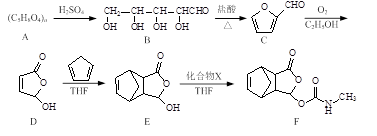
��1��������D�к��������ŵ�����Ϊ ��
��2������˵����ȷ����_________
��3��C������������ͭ��Ӧ�Ļ�ѧ����ʽΪ ��
��4��д��ͬʱ��������������E��һ��ͬ���칹��Ľṹ��ʽ�� ��
I.�˴Ź���������4���壻 ��.�ܷ���������Ӧ��ˮ�ⷴӦ�� ��.����FeCl3��Һ������ɫ��Ӧ��
��5����֪E��X��FΪ�ӳɷ�Ӧ��������X�Ľṹ��ʽΪ ��
��6����֪��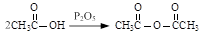 ��������
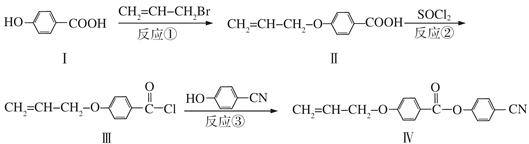
��������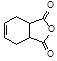 �Ǻϳɿ�����ҩ������Τ���м��壬��������
�Ǻϳɿ�����ҩ������Τ���м��壬��������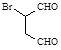 ����������ȥ������һϵ�з�Ӧ���ٺ�ԭ��M�ϳɡ�
����������ȥ������һϵ�з�Ӧ���ٺ�ԭ��M�ϳɡ�
����M�Ľṹ��ʽΪ
�����е���ȥ��Ӧ�Ļ�ѧ����Ϊ��
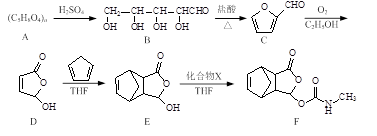
��1��������D�к��������ŵ�����Ϊ ��
��2������˵����ȷ����_________
| A��1 mol B������4 mol CH3COOH����������Ӧ |
| B��C�ķ���ʽΪC5H4O2 |
| C��D��E�ķ�Ӧ�Ǽӳɷ�Ӧ |
| D��E���Է�����ȥ��Ӧ��F����HCl��Ӧ |
��4��д��ͬʱ��������������E��һ��ͬ���칹��Ľṹ��ʽ�� ��
I.�˴Ź���������4���壻 ��.�ܷ���������Ӧ��ˮ�ⷴӦ�� ��.����FeCl3��Һ������ɫ��Ӧ��
��5����֪E��X��FΪ�ӳɷ�Ӧ��������X�Ľṹ��ʽΪ ��
��6����֪��
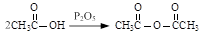 ��������
��������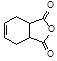 �Ǻϳɿ�����ҩ������Τ���м��壬��������
�Ǻϳɿ�����ҩ������Τ���м��壬��������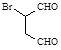 ����������ȥ������һϵ�з�Ӧ���ٺ�ԭ��M�ϳɡ�
����������ȥ������һϵ�з�Ӧ���ٺ�ԭ��M�ϳɡ�����M�Ľṹ��ʽΪ
�����е���ȥ��Ӧ�Ļ�ѧ����Ϊ��
��1���������ǻ� ��2�֣�
��2��ABCD ��2�֣�ȫ�Բŵ÷֣�
��3��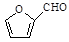 ��2Cu(OH)2��NaOH
��2Cu(OH)2��NaOH
 ��Cu2O����3H2O ��3�֣�
��Cu2O����3H2O ��3�֣�
����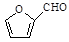 ��2Cu(OH)2
��2Cu(OH)2
 ��Cu2O����2H2O ��
��Cu2O����2H2O ��
��4��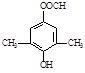 ��
��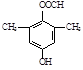 ��2�֣�
��2�֣�
��5��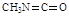 ��2�֣�
��2�֣�
��6����CH2=" CH-CH=" CH2��2�֣�
��HOOCCH(Br)CH2COOH + 3NaOH��NaOOCCH="CHCOONa" + NaBr +3H2O (3��) (����:���ȡ���) ��û������ֻ��1�֣�
��2��ABCD ��2�֣�ȫ�Բŵ÷֣�
��3��
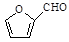 ��2Cu(OH)2��NaOH
��2Cu(OH)2��NaOH
 ��Cu2O����3H2O ��3�֣�
��Cu2O����3H2O ��3�֣�����
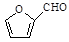 ��2Cu(OH)2
��2Cu(OH)2
 ��Cu2O����2H2O ��
��Cu2O����2H2O ����4��
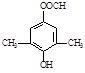 ��
��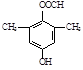 ��2�֣�
��2�֣���5��
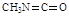 ��2�֣�
��2�֣���6����CH2=" CH-CH=" CH2��2�֣�
��HOOCCH(Br)CH2COOH + 3NaOH��NaOOCCH="CHCOONa" + NaBr +3H2O (3��) (����:���ȡ���) ��û������ֻ��1�֣�
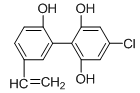
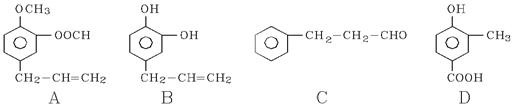
�����������1������D�Ľṹ��ʽ��֪��������D�к��������ŵ�����Ϊ�������ǻ�
��2��B��������4�����ǻ�������1 mol B������4 mol CH3COOH����������Ӧ��A��ȷ������C�Ľṹ��ʽ�ɵ�C�ķ���ʽΪC5H4O2 ��D��E�Ĺ��̷����˻��ӳɷ�Ӧ���γ�һ���µĻ���C��ȷ��E�����еĴ��ǻ���λ����H�����Կ��Է�����ȥ��Ӧ����F�����к����������������������������¶���ˮ�⣬���Կ�����HCl��Ӧ��D��ȷ����ѡABCD��
��3��C�к���ȩ�����ܺ����Ƶ�������ͭ����Һ��Ӧ����C������������ͭ��Ӧ�Ļ�ѧ����ʽΪ
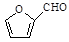 ��2Cu(OH)2��NaOH
��2Cu(OH)2��NaOH
 ��Cu2O����3H2O
��Cu2O����3H2O��4���ܷ���������Ӧ��ˮ�ⷴӦ����˵�������к���������ȩ��������FeCl3��Һ������ɫ��Ӧ��˵�������к��з��ǻ�������Ϊ�˴Ź���������4���壬����Ӧ���Ǽ����γɵ���������ܵĽṹ��ʽ��
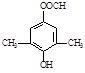
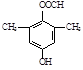
��4������EF�Ľṹ��ʽ������ԭ���غ��֪���÷�ӦӦ���Ǽӳɷ�Ӧ������X�Ľṹ��ʽӦ����CH3N=C=O
��5��
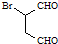 �ɷ�������������
�ɷ�������������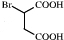 ��Ȼ������ȥ��Ӧ����
��Ȼ������ȥ��Ӧ����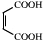 ��Ȼ����ȡ����Ӧ������
��Ȼ����ȡ����Ӧ������ �����ɵ�������������
�����ɵ�������������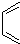 ��������D��E�Ļ��ӳɷ�Ӧ����Ŀ������ԣ�
��������D��E�Ļ��ӳɷ�Ӧ����Ŀ������ԣ���M�Ľṹ��ʽΪCH2=" CH-CH=" CH2
�ڷ�Ӧ�����е���ȥ��ӦΪ��HOOCCH(Br)CH2COOH + 3NaOH��NaOOCCH="CHCOONa" + NaBr +3H2OΪ

��ϰ��ϵ�д�
�����Ŀ

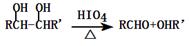
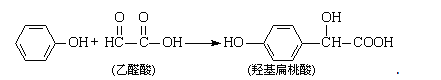


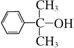 Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��______________��
Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��______________��