��Ŀ����
��14�֣���ѧ��һ����ʵ��Ϊ������ѧ�ƣ�����ʵ�������������ȷ����_ _
�� Ϊʹϡ�����п��ȡ���������ʼӿ죬����ϡ�����м�����ͭ˿��������п��
�� ��������ƽ��ȡ10. 4 gʳ��ʱ����ʳ�η��������е�ֽƬ�ϳ���
�� ����һ�����ʵ���Ũ�ȵ���Һʱ������ƿϴ�Ӻ�δ���и���
�� �Է�̪��ָʾ�����ñ�Ũ�ȵ�����ζ�NaOH��Һ������Һ���dz��ɫʱ��ֹͣ�ζ�
�� �ⶨ��Һ��pHʱ���ýྻ������IJ�����պȡ������Һ��������ֽ�в�������ɫ�������ɫ�����ն���
��.201 1��3��12�գ��ձ��������¸�����й©����Ҫй©��Ϊ��131�;�137����
�����������ÿ�����һƬ��Ƭ������һ����Ԥ�����á��ҹ����ֵ������������ӵ�ʳ������Ϊ���������й�ר��ָ����Ե�����Ԥ�������Ե��������ã����ҹ����ηḻ��������������Ƭ�͵��������Ϣ��
��Ƭ����Ҫ�ɷ��ǵ⻯�أ�ÿƬ��100���˵ĵ⣬���˷���ÿ���һƬ��
���Σ��ӵ�ʳ�Σ���Ҫ�ɷ����Ȼ��ơ�����أ�ÿ����ʳ�����е⺬��Ϊ20��30���ˡ�
KIO3���õ�ⷨ�Ƶã���ʯīΪ�����������Ϊ��������һ���¶Ⱥ͵����µ��KI��
Һ���ܷ�Ӧ����ʽΪ��KI+3H2O====KIO3 +3H2����
Na2S2O3����I2��Ӧ��I2+2S2O32-����ɫ����2I��+ S4O62-����ɫ�����ش��������⣺
��1���⻯�ء�������еĵ��ǵ�127������127�͵�131��ԭ�ӽṹ�в���ͬ���� ����ÿ����ʳ�����е⺬��Ϊ20���˼ƣ���ÿ���____�����β��൱�ڷ���һƬ��Ƭ��˵�����Ե�����Ԥ�������Ե� ����С������ޡ������á�
��2����ⷨ��KIO3�У������ĵ缫��ӦʽΪ_ ___����������pHֵ �����������С���������䡱����
��3��Ϊ��ֹ�����̷��˻����ۼ�ð�ӵ�ʳ�Σ��йز��ż�ǿ�˼�⡣�����Ƕ�ij���г��۵ļӵ⾫���εļ����̣�
��ȡ��ʳ����Ʒ100.0 g��������������ˮ����ʹ������ȫ�ܽ⣬Ȼ������������ữ��
����- KI��Һ�������Һ����ɫ���йط�Ӧ�����ӷ���ʽΪ ��
����0.010mol��L��1��Na2S2O3��Һ�ζ�������ȥNa2S2O3��Һ12.00mLʱ��ɫ�պ���ȥ��
�üӵ�ʳ����KIO3�ĺ���Ϊ mg/kg���ɴ˿��ж���ʳ��Ϊ ����ϸ��ϸ���Ʒ��
�٢ۢݣ�2�֣�
��.��1����������1�֣���5��1�֣����ޣ�1�֣�
��2��I- +3 H2O-6 e-= IO3-+6 H+ ��2�֣�������2�֣�
��3����IO3- + 6 H++5 I- =3 I2 + 3 H2O ��2�֣�����42.8��2�֣����ϸ�1�֣�
�����������鳣���Ļ�ѧ����ʵ��������йؼ��㡣
�ٿ��Թ���ԭ��أ�п�Ǹ������ӿ췴Ӧ���ʣ���ȷ��������ƽ��ȡ��ԭ�����������룬�ڲ���ȷ������һ�����ʵ���Ũ����Һʱ������ƿ����Ҫ�������ȷ���к͵ζ�ʵ������Һ��ɫ�����仯��Ӧ���ڰ�����ڲ���ɫΪ�����ܲ���ȷ���ⶨ��Һ��pHʱ����ֽ����������ʪ�����Ԣ���ȷ��
��.��1�� ��127�͵�131����ͬλ�أ���������ͬ������������ͬ�����ݵ�ԭ���غ��֪������ʳ�ε�������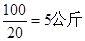 ����˿��Ե�����Ԥ�������Ե��Dz������õġ�
����˿��Ե�����Ԥ�������Ե��Dz������õġ�
��2��������������ʧȥ���ӣ�������Һ�еĵ�����ʧȥ���ӣ����������ɵ���أ��缫��ӦʽΪI- +3 H2O-6 e-= IO3-+6 H+�������õ����ӣ�������ԭ��Ӧ������������������ŵ磬�缫��ӦʽΪ2H+��2e����H2���������ӷŵ磬�ƻ���������Χˮ�ĵ���ƽ�⣬�Ӷ�ʹ������Χ��Һ�ļ�����ǿ��
��3����������Һ�У�����غ͵⻯�ط���������ԭ��Ӧ�����ɵ��ʵ⣬����ʽΪIO3- +
6 H++5 I- =3 I2 + 3 H2O������I2+2S2O32-����ɫ����2I��+ S4O62-����ɫ����֪����غ�Na2S2O3�����ʵ���֮����1�U6�����Ե���ص�������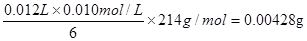 ��4.28mg���ӵ�ʳ����KIO3�ĺ���Ϊ
��4.28mg���ӵ�ʳ����KIO3�ĺ���Ϊ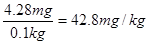 �����к����������
��������������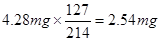
������ÿ����ʳ�����е⺬��Ϊ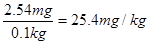 ������Ǻϸ��Ʒ��
������Ǻϸ��Ʒ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�