��Ŀ����
����11�֣���ͼΪij��ѧ��ȤС����ж����ѽ��ʵ�����̡���ע��CuO�ܽ���������CO2��H2O��G����װ��������أ�ʡ�ԡ���

��ͼ���Ӻ�װ�ú�����е�ʵ������У��ٸ�D��Gװ�ü��ȣ��ڼ������װ�õ������ԣ����ų�װ���еĿ�������
��1���������������Ⱥ�˳��������������������������
��2����Ҫ˵���ſ����ķ�����֤���������ž��ķ�������������������������������
��3��Bװ���������������������������������������
��4������Eװ���еĻ�����ٰ���������ʵ�飺

�ٷ������I��II�ķ����ǣ�I��������������������II����������������
��Na2SO3��Һ�������ǣ������ӷ���ʽ��ʾ��
����������������������������������������������������������������
��5���ٶ�������ȫ�ѽ⣬������װ���е���������ȫ��Ӧ������E��F��װ�õ��������ȷ�Ӧǰ������1.82g��Gװ���й�������������4.16g��������ѽ�����У�n(CH4):n(C2H6)=

��ͼ���Ӻ�װ�ú�����е�ʵ������У��ٸ�D��Gװ�ü��ȣ��ڼ������װ�õ������ԣ����ų�װ���еĿ�������
��1���������������Ⱥ�˳��������������������������
��2����Ҫ˵���ſ����ķ�����֤���������ž��ķ�������������������������������
��3��Bװ���������������������������������������
��4������Eװ���еĻ�����ٰ���������ʵ�飺

�ٷ������I��II�ķ����ǣ�I��������������������II����������������
��Na2SO3��Һ�������ǣ������ӷ���ʽ��ʾ��
����������������������������������������������������������������
��5���ٶ�������ȫ�ѽ⣬������װ���е���������ȫ��Ӧ������E��F��װ�õ��������ȷ�Ӧǰ������1.82g��Gװ���й�������������4.16g��������ѽ�����У�n(CH4):n(C2H6)=
��1���ڢۢ١�������1�֣�
��2����K��ʹ����������������װ�ã���װ��G��ĩ���ռ�һС�Թ������ס�ܿڣ��������棬�ɿ���ָ����û�б�������������ž���2�֣�
��3��ͨ���۲����ݣ�����K�������������٣�2�֣�
��4����I����Һ��II������1�֣�
��SO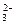 +Br2+H2O=SO
+Br2+H2O=SO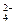 +2Br��+2H+��2�֣�
+2Br��+2H+��2�֣�
��5��3:2��2�֣�
��2����K��ʹ����������������װ�ã���װ��G��ĩ���ռ�һС�Թ������ס�ܿڣ��������棬�ɿ���ָ����û�б�������������ž���2�֣�
��3��ͨ���۲����ݣ�����K�������������٣�2�֣�
��4����I����Һ��II������1�֣�
��SO
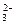 +Br2+H2O=SO
+Br2+H2O=SO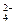 +2Br��+2H+��2�֣�
+2Br��+2H+��2�֣���5��3:2��2�֣�
��

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
 ���ķ���ʽ��______________��1mol����������������ȡ����Ӧ���������е���ȫ����ȡ�����������������ʵ�����______mol��
���ķ���ʽ��______________��1mol����������������ȡ����Ӧ���������е���ȫ����ȡ�����������������ʵ�����______mol�� ��������ʵ���֮�Ⱥ������ȡ�
��������ʵ���֮�Ⱥ������ȡ�