��Ŀ����
��10�֣���A��B��C��D��E���ֳ���������������������е�һ�ֻ����γɵģ��������У���������������̼����������������������У������ӡ������ӡ�ͭ���ӡ������ӡ�
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٷֱ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ��ֻ��B��CΪ��ɫ(����ɫ�ܲ���)��
���ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬA�зų���ɫ���壬C��D�в�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��������ʵ����գ�
(1) д��B��D�Ļ�ѧʽ��B____________��D___________��
(2)����1 mol A����Һ�뺬1 mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ļ�ѧʽΪ____________��
(3)��A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ ��
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٷֱ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ��ֻ��B��CΪ��ɫ(����ɫ�ܲ���)��
���ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬA�зų���ɫ���壬C��D�в�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��������ʵ����գ�
(1) д��B��D�Ļ�ѧʽ��B____________��D___________��
(2)����1 mol A����Һ�뺬1 mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ļ�ѧʽΪ____________��
(3)��A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ ��
(4��) (1)KNO3 CuSO4
(2)Na2CO3
��4�֣���3��2HCO3-+Ca2++2OH-====CaCO3��+ CO32-+2H2O
(2)Na2CO3
��4�֣���3��2HCO3-+Ca2++2OH-====CaCO3��+ CO32-+2H2O
����������⣺�ٽ���������ˮ��DΪ��ɫ��Һ��˵��D�к�������Cu2+���ڽ�E��Һ���˵�C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣬���������ӿ��Կ�����ӦΪAl3+��OH-�ķ�Ӧ����E�к���OH-��C�к���Al3+���۽�����ɫ��Ӧ��ֻ��B��CΪ��ɫ������ɫ�겣������˵��ֻ��B��C�к���K+�����ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬA�зų���ɫ���壬��A����HCO3-��C��D�в�����ɫ�����ó���ΪBaSO4��˵��C��D�к���SO42-���ݽ�B��D����Һ��ϣ�δ���������������ɣ�˵������û�з����κη�Ӧ����BΪKNO3����������A��B��C��D��E���ֳ���������ֱ�Ϊ��NaHCO3��KNO3��KAl��SO4��2��CuSO4��NaOH��
��1��B��D������ֱ�Ϊ��KNO3��CuSO4���ʴ�Ϊ��KNO3��CuSO4��
��2����NaHCO3��NaOH�����ʵ�����Ӧ����̼���ƺ�ˮ�����Է�Ӧ����Һ���ɣ��õ�һ�ֻ�����ΪNa2CO3���ʴ�Ϊ��Na2CO3����3������NaHCO3����������ʯ��ˮ�������ߵ����ʵ���֮��Ϊ2��1������ʽΪ��2NaHCO3+Ca��OH��2=CaCO3��+2H2O+Na2CO3�����ӷ���ʽΪ��Ca2++2HCO3-+2OH-=CaCO3��+2H2O+CO32-���ʴ�Ϊ��Ca2++2HCO3-+2OH-=CaCO3��+2H2O+CO32-��
������������Ҫ�������ӵļ��飬�����֪ʶ��϶࣬��Ŀ�ѶȽϴ�ע������ӷ�Ӧ����������ӹ���ȽǶ�˼������д���ӷ���ʽʱע�����Ĺ�ϵ��

��ϰ��ϵ�д�
�����Ŀ
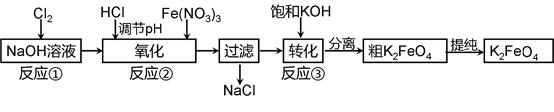
 4Fe(OH)3����8OH��+3O2��,��K2FeO4������ˮ�����е������� ��
4Fe(OH)3����8OH��+3O2��,��K2FeO4������ˮ�����е������� ��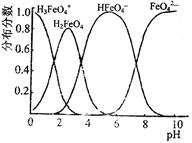
 Na++H++SO42һ
Na++H++SO42һ