��Ŀ����
���й���ȼ���ȵ���������ȷ����(����)
| A����֪a g��ϩ������ȼ��ʱ����1 mol CO2��Һ̬ˮ���ų�b kJ�����������ʾ��ϩȼ���ȵ��Ȼ�ѧ����ʽΪ2C2H4(g)��6O2(g)=4CO2(g)��4H2O(l) ��H����4b kJ·mol��1 |
B�����״�����ת��Ϊ�������Ȼ�ѧ����ʽ��CH3OH(g)��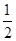 O2(g)=CO2(g)��2H2(g) ��H����192.9 kJ·mol��1����CH3OH(g)��ȼ����Ϊ192.9 kJ·mol��1 O2(g)=CO2(g)��2H2(g) ��H����192.9 kJ·mol��1����CH3OH(g)��ȼ����Ϊ192.9 kJ·mol��1 |
| C��H2(g)��ȼ������285.8 kJ·mol��1����2H2O(g)=2H2(g)��O2(g) ��H����571.6 kJ·mol��1 |
D�������ǵ�ȼ������2800kJ·mol��1����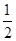 C6H12O6(s)��3O2(g)=3CO2(g)��3H2O(l) ��H����1400kJ·mol��1 C6H12O6(s)��3O2(g)=3CO2(g)��3H2O(l) ��H����1400kJ·mol��1 |
D
���������A����ϩ��ȼ����Ϊ1mol��ϩ���ȼ�շų�������������B���״���ȼ������1mol�״����ȼ�շų������������״����ȼ�յIJ��ﲻ����������ˮ������C��������ȼ������1mol�������������ȼ�շų���������������ʽ2H2O(g)=2H2(g)��O2(g)��ʾ����ˮ�ķֽⷴӦ������D����ȷ��

��ϰ��ϵ�д�
�����Ŀ
 (NH4)2 SO4��Һ����μ���0.2000 mol
(NH4)2 SO4��Һ����μ���0.2000 mol

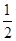 N2O4(g)=
N2O4(g)=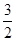 N2(g)+2H2O(g) ��H="-1076.7" kJ��mol-1
N2(g)+2H2O(g) ��H="-1076.7" kJ��mol-1