��Ŀ����
����Ŀ���뼰�仯�����Ӧ�������汻���ӡ�
��l������Ҫ�ĺ������������ʯ����2������Cr��������ʯ��Ϊ��ĸ�̡���̬Crԭ�Ӽ۵��ӵĹ����ʾʽΪ_____________________��
��2�����������������Ԫ���������ơ������й��������������ȷ����_______�����ţ���
A��������p������Ԫ�� B���縺�Զ���þ��
C����һ�����ܶ���þ�� D.�Ȼ����ˮ��ҺpH��С��7
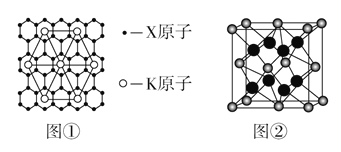
��3���롢�����嶼���ɽ���ԭ�����ò�����ά�ռ�ѻ����ɣ����ܶѻ���������۵�(155lK)�������۵�(930K)�ߣ�ԭ����__________________________��
��4���Ȼ�������̬ʱ����BeC12���ӣ�a���Ͷ��۷��ӣ�BeCl2)2(b)����̬ʱ���������ͼ��ʾ����״�ṹ��c����

��a����__________������ԡ��Ǽ��ԡ������ӡ�
�ڶ��۷��ӣ�BeCl2)2��Beԭ�ӵ��ӻ���ʽ��ͬ��������ԭ�Ӷ���ͬһƽ���ϡ�b �ĽṹʽΪ________________________________ �������λ������
��c��Beԭ�ӵ��ӻ���ʽΪ__________��
���Ȼ��뾧���д��ڵ���������_____�����ţ���
A�����»��� B. �Ҽ� C.���Լ� D���Ǽ��Լ� E�����Ӽ�
��5��BeO������������ͼ��ʾ��

��BeO������ܶ�Ϊdg/cm3��������a =______nm ���г�����ʽ���ɣ���
���𰸡� ![]() B��D Beԭ�Ӱ뾶��Alԭ�Ӱ뾶С�������Ը�ǿ �Ǽ���
B��D Beԭ�Ӱ뾶��Alԭ�Ӱ뾶С�������Ը�ǿ �Ǽ��� ![]() sp3 A��B��C
sp3 A��B��C ![]()
��������(l)Crԭ��Ϊ24��Ԫ�أ������������ȫ�����ȶ��ṹ����̬ԭ�Ӽ۲�����Ų�ʽΪ3d54s1,���̬Crԭ�Ӽ۵��ӵĹ����ʾʽΪ![]() ��
��
(2)A��������s��Ԫ�أ���������p������Ԫ�أ���A����B������Խ���ã������ԽС��Mg��Be��Al���ã����������縺�Զ���þ��B��ȷ��C��Be�ĵ�һ�����ܱ�þ��Mg��s���ȫ��������һ�����ܱ�Al��C����D.Be2+��Al3+����Һ�о�ˮ�⣬���Ȼ����ˮ��ҺpH��С��7����D��ȷ����ΪBD��
(3)��ΪBeԭ�Ӱ뾶��Alԭ�Ӱ뾶С�������Ը�ǿ��������۵�������۵����
(4)�Ȼ�������̬ʱ����BeC12����(a)�Ͷ��۷���(BeCl2)2(b)����̬ʱ���������ͼ��ʾ����״�ṹ(c)��
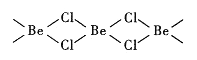
��BeCl2��Beԭ�ӳ�2��Be-Cl��Beԭ�ӵ��ӻ������Ϊ2����Beԭ�Ӳ�ȡsp�ӻ���ʽ����ֱ���ͣ����ڷǼ��Է�����
���ڶ��۷���(BeCl2)2��Beԭ���пչ����Clԭ���й¶Ե��ӣ�����λ���е��Ӷ�����ԭ���ṩ��b �ĽṹʽΪ![]() ��
��
������пչ�����Ⱦ��йµ��Ӷԣ����Զ����(BeCl2)N�ṹ��ͼΪ![]() �м۲���ӶԸ���=4������ԭ������sp3�ӻ���
�м۲���ӶԸ���=4������ԭ������sp3�ӻ���
�� �Ȼ��뾧���Ƿ��Ӿ��壬���Ӽ��з��»�������������Be��Cl���Լ���Ҳ�������������ڷǼ��Լ������Ӽ����ʴ�ΪABC��
(5)BeO����������ͼ��ʾ������Be2+��ĿΪ4��O2-��ĿΪ8![]() +6��
+6��![]() =4�������к���4��BeO������������Ϊ
=4�������к���4��BeO������������Ϊ![]() g���辧���ı߳�Ϊanm���������Ϊ(a��10-7)3cm3���������ܶ�dg/cm3=
g���辧���ı߳�Ϊanm���������Ϊ(a��10-7)3cm3���������ܶ�dg/cm3=![]() g��(a��10-7)3cm3����a=
g��(a��10-7)3cm3����a=![]() nm��
nm��

����Ŀ��[��ѧ��ѡ��3�����ʽṹ������]
��п��ZnS���������������Լ���ӫ���塢���ģ�����ʪ�������ױ�����ΪZnSO4���ش��������⣺
��д����̬Znԭ�ӵļ۵����Ų�ʽ__________����̬Sԭ�Ӻ���δ�ɶԵ�����Ϊ_____��
��ZnSO4������Ԫ�صĵ縺���ɴ�С��˳��Ϊ_____________________��SO42�������幹��Ϊ________________������S���ӻ��������Ϊ_________��
������п���ڰ�ˮ������[Zn(NH3)4]SO4��Һ��[Zn(NH3)4]SO4��Һ�������ڵ�������������___________��
a.���Ӽ� b.���ۼ� c.��λ�� d.���»��� e.���
�ȸ�������п±������۵���ܽ��ԣ��ж�ZnF2���������Ϊ___________������ZnCl2��ZnBr2��ZnI2�۵����������ԭ��________________��
ZnF2 | ZnCl2 | ZnBr2 | ZnI2 | |
�۵�/�� | 872 | 275 | 394 | 446 |
���Ҵ����������ܽ��� | ���� | �ܽ� | �ܽ� | �ܽ� |
������ZnS������ܶ�Ϊ��g��cm-3���侧���ṹ��ͼ��S2����Χ�Ⱦ����������Zn2+��S2������Ϊ______��______��ZnS�����еľ�������a=________nm(�г�����ʽ)��