��Ŀ����
����ʵ����Ʋ��ܻ�óɹ����ǣ� ��
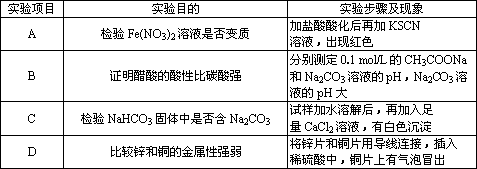
| ʵ����Ŀ | ʵ��Ŀ�� | ʵ�鲽�輰���� |
| A | ����Fe(NO3)2��Һ�Ƿ���� | �������ữ���ټ�KSCN��Һ�����ֺ�ɫ |
| B | ֤����������Ա�̼��ǿ | �ֱ�ⶨ0.1mol/L��CH3COONa��Na2CO3��Һ��pH��Na2CO3��Һ��pH�� |
| C | ����NaHCO3�������Ƿ�Na2CO3 | ������ˮ�ܽ���ټ�������CaCl2��Һ���а�ɫ���� |
| D | �Ƚ�п��ͭ�Ľ�����ǿ�� | ��пƬ��ͭƬ�õ������ӣ�����ϡ�����У�ͭƬ��������ð�� |
A

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
��ҵβ���е�������ͨ�����ð������շ�����ԭ����NH3��NOx�ڴ��������·�Ӧ�����������ʣ�ijУ�С��ͬѧ��������װ�úͲ���ģ�ҵ�ϵ�������Ĵ������̣�
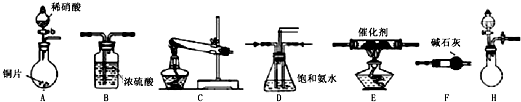
��̽����ȡNH3�ķ���
��1��������װ���У�H�ܿ��١������ȡNH3��װ������Ҫ���ӵķ�Ӧ�Լ�Ϊ ��
��2��Ϊ̽�����õ�ʵ��Ч�����С��ͬѧ��������Cװ������ȡ�������ڿ���ʵ��������ͬ������£�����±���ʵ�����ݣ�
�����������ݣ�����Ϊ���ַ�����ȡ������Ч����ã�����ţ����Ӹ÷���ѡ���ԭ�Ϸ�������Ч���õĿ���ԭ���� ��
��ģ��β������
�С��ͬѧѡ����������װ�ã�������˳�����ӳ�ģ��β������װ�ý���ʵ�飮
��1���������װ����ѡ������Ϊ�����Ľ��в��䣨��ѡװ�ò����ظ�����

��2��A�з�Ӧ�����ӷ���ʽΪ ��
��3��Dװ�õ������У�ʹ�����Ͼ��ȡ����������ٶȡ� ��
��4��Dװ���е�Һ�廹�ɻ��� ������ţ���
a��H2O b��CCl4 c��ŨH2SO4 d��CuSO4��Һ
��5����С��ͬѧ����Ƶ�ģ��β������װ���л�����һ�����Ե�ȱ���� ��

��̽����ȡNH3�ķ���
��1��������װ���У�H�ܿ��١������ȡNH3��װ������Ҫ���ӵķ�Ӧ�Լ�Ϊ
��2��Ϊ̽�����õ�ʵ��Ч�����С��ͬѧ��������Cװ������ȡ�������ڿ���ʵ��������ͬ������£�����±���ʵ�����ݣ�
| �Լ������� | �����Լ� | NH3�����mL�� | |
| a | 6.0g Ca��OH��2�������� | 5.4g NH4Cl | 1344 |
| b | 5.4g ��NH4��2SO4 | 1364 | |
| c | 6.0g NaOH�������� | 5.4g NH4Cl | 1568 |
| d | 5.4g ��NH4��2SO4 | 1559 | |
| e | 6.0g CaO�������� | 5.4g NH4Cl | 1753 |
| f | 5.4g ��NH4��2SO4 | 1792 | |
��ģ��β������
�С��ͬѧѡ����������װ�ã�������˳�����ӳ�ģ��β������װ�ý���ʵ�飮
��1���������װ����ѡ������Ϊ�����Ľ��в��䣨��ѡװ�ò����ظ�����

��2��A�з�Ӧ�����ӷ���ʽΪ
��3��Dװ�õ������У�ʹ�����Ͼ��ȡ����������ٶȡ�
��4��Dװ���е�Һ�廹�ɻ���
a��H2O b��CCl4 c��ŨH2SO4 d��CuSO4��Һ
��5����С��ͬѧ����Ƶ�ģ��β������װ���л�����һ�����Ե�ȱ����
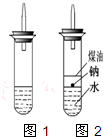 ijʵ��С�����ѧ�α����Ѿ�ѧ�������������ķ�Ӧ�������о���д�����������������������ķ�Ӧ��
ijʵ��С�����ѧ�α����Ѿ�ѧ�������������ķ�Ӧ�������о���д�����������������������ķ�Ӧ��