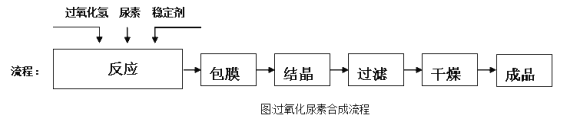
��Ŀ����
����Ŀ����֪Ũ�������Ҵ��Ļ��Һ���Ⱥ�ɲ�����ϩ.Ϊ̽����ϩ����ļӳɷ�Ӧ,��ͬѧ��Ʋ�����������ʵ�飺�����ɵ�����ͨ����ˮ�У�������Һ��ɫ����֤����ϩ����ˮ�����˼ӳɷ�Ӧ��
��ͬѧ�ڼ�ͬѧ��ʵ���У�������ɫ�����Һ������������ӣ��Ʋ����Ƶõ���ϩ�л�������������ԭ���������ʣ��ɴ�����������ȳ�ȥ֮��������ˮ��Ӧ��
��ش��������⣺
��1����ͬѧʵ������ˮ��ɫ����Ҫ����ʽΪ_________________________________________________��
��2����ͬѧ��Ƶ�ʵ��_____________________(���ܻ���)��֤��ϩ����ˮ�����ӳɷ�Ӧ,������_____________________________________��
A��ʹ��ˮ��ɫ�ķ�Ӧδ���Ǽӳɷ�Ӧ��
B��ʹ��ˮ��ɫ�ķ�Ӧ���Ǽӳɷ�Ӧ��
C��ʹ��ˮ��ɫ������δ������ϩ��
D��ʹ��ˮ��ɫ�����ʾ�����ϩ��
��3����ͬѧ�Ʋ���ϩ�бض����е�һ��������SO2��������________________________����֤,������ˮ��Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
��4��Ϊ��֤��һ��Ӧ�Ǽӳɶ�����ȡ������ͬѧ�������pH ��ֽ�����Է�Ӧ����Һ�����ԣ�������______________________________________________________________________________��
���𰸡�  ���� a c Ʒ����Һ 2H2O + Br2 + SO2 = 2HBr + H2SO4 �ӳɷ�Ӧ��HBr���ɣ���ȡ����Ӧ����HBr����
���� a c Ʒ����Һ 2H2O + Br2 + SO2 = 2HBr + H2SO4 �ӳɷ�Ӧ��HBr���ɣ���ȡ����Ӧ����HBr����
��������(1)��ϩ����ˮ�����ӳɷ�Ӧ����ˮ��ɫ����Ҫԭ�û�ѧ����ʽΪ��CH2=CH2��Br2��CH2BrCH2Br ��(2)���Ƶõ���ϩ�к���������ԭ���������ʣ���ԭ������������ˮ����������ԭ��Ӧ���Ӷ�ʹ��ˮ��ɫ�����ͬѧ��Ƶ�ʵ�鲻����֤��ϩ����ˮ�����ӳɷ�Ӧ,������ʹ��ˮ��ɫ�ķ�Ӧδ���Ǽӳɷ�Ӧ��ʹ��ˮ��ɫ������δ������ϩ����AC�� (3)��ͬѧʵ������ɫ�����Һ����SO42- �������֪��ϩ�����л����л�ԭ������SO2 ��������ΪŨ���������ˮ�ԣ���ʹ�����Ҵ���ˮ̿�����ڼ���ʱ��̼��Ũ���ᷢ��������ԭ��Ӧ����SO2 ���塣���Ƶõ���ϩ����ͨ��Ʒ����Һ�У���Ʒ����Һ��ɫ��֤���Ƶõ���ϩ�����к��� SO2�� SO2��ʹ��ˮ��ɫ����Ӧ�Ļ�ѧ����ʽΪ��2H2O + Br2 + SO2 = 2HBr + H2SO4�� (4)����ˮ����ϩ�����ӳɷ�Ӧ������1��2 �����������⣬һ������ ����HBr���Ӷ�ʹ��Һ����������ǿ���ʿ���PH��ֽ��֤��