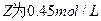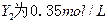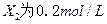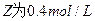��Ŀ����
(10��)����ЧӦ����Դ��ȱ ���������ν��ʹ����е�CO2���������Կ������������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)��3H2(g)?
���������ν��ʹ����е�CO2���������Կ������������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)��3H2(g)?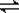 ?CH3OH(g)��H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
?CH3OH(g)��H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
(1)���ڸ÷�Ӧ������˵���У���ȷ����________��
A����H>0����S>0
B����H>0����S<0
C����H<0����S<0
D����H<0����S>0
(2)�÷�Ӧƽ�ⳣ��K�ı���ʽΪ________________________________��
(3)�¶Ƚ��ͣ�ƽ�ⳣ��K________(����������䡱��С��)��
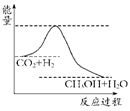
(4)Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(H2)________mol��L��1��min��1��
(5)���д�ʩ����ʹn(CH3OH)/n(CO2)�������________��
A�������¶�
B���������
C����H2O(g)����ϵ�з���
D���ٳ���1 mol CO2��3 mol H2
E������He(g)��ʹ��ϵ��ѹǿ����
 ���������ν��ʹ����е�CO2���������Կ������������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)��3H2(g)?
���������ν��ʹ����е�CO2���������Կ������������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)��3H2(g)?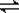 ?CH3OH(g)��H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
?CH3OH(g)��H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
(1)���ڸ÷�Ӧ������˵���У���ȷ����________��
A����H>0����S>0
B����H>0����S<0
C����H<0����S<0
D����H<0����S>0
(2)�÷�Ӧƽ�ⳣ��K�ı���ʽΪ________________________________��
(3)�¶Ƚ��ͣ�ƽ�ⳣ��K________(����������䡱��С��)��
(4)Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(H2)________mol��L��1��min��1��

(5)���д�ʩ����ʹn(CH3OH)/n(CO2)�������________��
A�������¶�
B���������
C����H2O(g)����ϵ�з���
D���ٳ���1 mol CO2��3 mol H2
E������He(g)��ʹ��ϵ��ѹǿ����
(1)C�� (2)��(3)���� (4)0.225 ��(5)CD
��

��ϰ��ϵ�д�
�����Ŀ
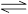 zC���ﵽƽ�⣬����д���пհף�
zC���ﵽƽ�⣬����д���пհף�
 NO2��CO ������˵����ȷ���ǣ� ��
NO2��CO ������˵����ȷ���ǣ� ��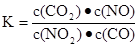
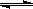 2AB2(g)������ӦΪ���ȷ�Ӧ�������и�ͼ����ʾ�ı仯������������ԭ������
2AB2(g)������ӦΪ���ȷ�Ӧ�������и�ͼ����ʾ�ı仯������������ԭ������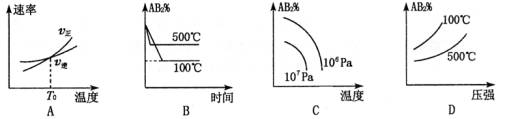
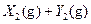
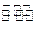
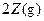 ��֪X1��Y2��Z����ʼŨ
��֪X1��Y2��Z����ʼŨ �ȷֱ�Ϊ
�ȷֱ�Ϊ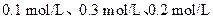 ����һ�������µ���Ӧ�ﵽƽ��ʱ�������ʵ�Ũ���п��ܣ� ��
����һ�������µ���Ӧ�ﵽƽ��ʱ�������ʵ�Ũ���п��ܣ� ��