��Ŀ����
1���ҹ�������þ��Դ�����ȫ���г���þ��80%���ϴ��й����ڣ�þ���Ͻ������������졢���ա���е���졢���µȲ�ҵ��Ӧ��Ѹ�ͷ�չ��Ԫ��þ�����ڱ��е�λ�õ�3���ڵڢ�A�壮����þ�Ͻ𱻴���Ӧ�����ƳɱʼDZ�������ǡ��������г���ܵȣ�������þ�Ͻ����Ӳ�ȴ���ʴ���ܶ�С���������ܣ�C��Mg����Ԫ�����γ�����������������Ϊ1��1�����ӻ�����˻���������ˮ��Ӧ����һ�����壬������ĽṹʽΪH-C��C-H��
���� ��������=���Ӳ���������Ԫ����������������������������þ�Ͻ����Ӳ�ȴ���ʴ���ܶ�С�ȷ�����ص㣬C��Mg��Ԫ���γ�����������������Ϊ1��1�����ӻ�����ΪMgC2������ˮ��Ӧ������Ȳ��
��� �⣺Ԫ��þ��������Ϊ12����ԭ�ӻ�̬ʱ�ĺ�������Ų�ʽΪ1s22s22p63s2��Ԫ�غ����������Ӳ㣬�����2�����ӣ���������=���Ӳ���������Ԫ������������������������������λ��Ϊ��3���ڵڢ�A�壬����þ�Ͻ𱻴���Ӧ�����ƳɱʼDZ�������ǡ��������г���ܵ�ʱ��Ҫ��������þ�Ͻ����Ӳ�ȴ���ʴ���ܶ�С�ȷ�����ص㣬C��Mg��Ԫ���γ�����������������Ϊ1��1�����ӻ�����ΪMgC2����ˮ��Ӧ���ɵ�����ΪC2H2��������ĽṹʽΪH-C��C-H��
�ʴ�Ϊ����3���ڵڢ�A�壻Ӳ�ȴ���ʴ���ܶ�С��H-C��C-H��
���� ���⿼����Ԫ�������ڱ���λ�õ��жϡ��Ͻ�����ʡ�̼��þ�����ʵȣ���ȷԪ��ԭ�Ӻ�����Ӳ�����������������������������������֮��Ĺ�ϵ�����ճ����Ͻ�������Լ�������Ϣ�ƶ�̼��þ��ˮ������ǽ����Ĺؼ������ض�ѧ���������⡢֪ʶǨ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

| Ԫ�� | �����Ϣ |
| X | XԪ�ؿ��γ���Ȼ����Ӳ���������� |
| Y | �䵥��Ϊ˫ԭ�ӷ��ӣ�������⻯���ˮ��Һ��ʹ��̪��� |
| Z | Z�Ƕ�������������ʧȥ���ӵ�Ԫ�� |
| M | M��һ��ͬλ�ص�������Ϊ34��������Ϊ18 |
| N | N�Ǿ����Ϻ�ɫ����Ľ������кܺõ���չ�ԡ������Ժ͵����� |
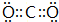 ��
����2����״���£���4.48L X�����������ͨ��1L 0.3mol•L-1 Z������������Ӧˮ�������Һ�з�����Ӧ�����ӷ���ʽΪ3OH-+2CO2=HCO3-+CO32-+H2O��
��3��Y���⻯��Y2H4�ǻ���ij���ȼ�ϣ���֪16.0g��Һ̬ȼ������������ȫȼ�����ɵ�����Һ̬ˮʱ���ų�312kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624 kJ•mol-1��
��4��Ԫ��N������������е������Լ�������Ӧ�����ɳ����ᣨHO2����N+HCl+O2�TNCl+HO2��HO2������һ���������Ҳ��һ�����ɻ������м��ߵĻ��ԣ������йظ÷�Ӧ��˵���������C��
A����������O2
B��HO2�ڼ��в����ȶ�����
C������������HO2
D.1mol N�μӷ�Ӧ����1mol���ӷ���ת��
��5��Y��Z��Ԫ���γɵĻ�����H�����������İ�ȫ���ң�ȡ6.5g H���ײ���ֽ��õ����ֵ��ʣ�����һ�����嵥�ʵ�����ڱ�״����Ϊ3.36L����H�Ļ�ѧʽΪNaN3�������ʵľ�������Ϊ���Ӿ��壮
| ��Ļ�ѧʽ ���볣��K | ������ H2S | ������ H2SO3 | ���� H2CrO4 | ���� HCN |
| K1 | 9.1��10-8 | 1.5��10-2 | 1.8��10-1 | 5.0��10-10 |
| K2 | 1.1��10-12 | 1.0��10-7 | 3.2��10-7 |
��2�������£�KCN��ˮ�ⳣ��KhΪ2.0��10-5��
��3�������£�Ũ����ͬ��K2S��K2SO3��K2CrO4����ˮ��Һ�У�ˮ����̶�������K2S��
��4�������£�0.1mol•L-1��������Һ������Ũ�ȴ�С����ΪH+��HS-��S2+��OH-����
��5��H2SO3�ڶ������볣��С�ڵ�һ�����볣��������Ҫԭ����H2SO3��һ����������������ӻ����Ƶڶ������룮
��6����֪�����£�Ksp��Ag2CrO4��=1.12��10-12��Ksp��BaCrO4��=1.17��10-10����Ũ�ȶ�Ϊ0.001mol•L-1�ĺ�Ag+��Ba2+����Һ�У������μ�ϡK2CrO4��Һ���Ȳ����ij����Ļ�ѧʽ��BaCrO4��
 CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�����
CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�������1����CO2��NH3Ϊԭ�Ϻϳɻ������ص���Ҫ��Ӧ���£�
��2NH3��g��+CO2��g��=NH2CO2NH4��s������H=-159.47kJ•mol-1
��NH2CO2NH4��s��=CO��NH2��2��s��+H2O��g������H=a kJ•mol-1
��2NH3��g��+CO2��g��=CO��NH2��2��s��+H2O��g������H=-86.98kJ•mol-1
��aΪ+72.49kJ��mol-1��
��2����ѧ��������ù�ҵ�����е�CO2��ȡ�״���CO2+3H2CH3OH+H2O��
�Ƶõ�CH3OH������ȼ�ϵ�ص�ȼ�ϣ�
����KOH�����У������ĵ缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��
�������ʵ�KOH�����õ��K2SO4��Һ�ķ����Ƶã���KOH��D���ڵõ��������ĵ缫��Ӧʽ�ǣ�4OH-+4e-=2H2O+O2��
��3������CO��H2��Ӧ�ɺϳ�CH3OCH3��
��֪��3H2��g��+3CO��g��=CH3OCH3��g��+CO2��g������H=-247kJ/mol
��һ�������µ��ܱ������У��÷�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��AE��
A�����¸�ѹ B��������� C�����������뺤��D������CO��Ũ�� E�������������
��4��CH3OCH3Ҳ����CH3OH�ϳɣ�
��֪��Ӧ2CH3OH��g��=CH3OCH3��g��+H2O��g������ij�¶��£���1L�ܱ������м���CH3OH����Ӧ��10����ʱ�ﵽƽ�⣬��ʱ��ø���ֵ�Ũ�������
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol•L-1�� | 0.01 | 0.2 | 0.2 |
�ڸ��¶��µ�ƽ�ⳣ��Ϊ400��
����ƽ��������������ټ���0.01mol CH3OH��0.2mol CH3OCH3����ʱ�����淴Ӧ���ʵĴ�С��v���� v�� �����������������=������
| A�� | Ca��CaO��Ca Cl2 | B�� | O2��CuO��Cu��OH��2 | ||
| C�� | C��CO2��Na2CO3 | D�� | NaOH��Na2CO3��NaCl |
 �ϳɰ���ҵ�����Ṥҵ�����Ṥҵ�ǻ�ѧ��ҵ����Ҫ��ɲ��֣���ش��������⣺
�ϳɰ���ҵ�����Ṥҵ�����Ṥҵ�ǻ�ѧ��ҵ����Ҫ��ɲ��֣���ش��������⣺