��Ŀ����
С�մ�(NaHCO3)��ʳƷ��ҵ��һ��Ӧ����㷺�����Ӽ���������ʾ��NaHCO3�����������п��ܲ��ַֽ��Na2CO3��ij��ѧ��ȤС��ͬѧ��ȡ28��95g��ҵNaHCO3��Ʒ���з��������þƾ��ƶ����ּ��ȣ���ȴ��Ƶ�������Ϊ21��2g������ȡһ����ͬ��������Ʒ����μ���ϡ������û�����ݲ���Ϊֹ�����ռ�����������ɱ�״���µ����Ϊ7��28L����������Ʒ�г���Na2CO3��NaHCO3�⣬�����������ʣ����������ȶ��Ҳ������ᷴӦ��ͨ������ش��������⣺
��1��д��NaHCO3���ȷֽ�Ļ�ѧ��Ӧ����ʽ_________________________��
��2�����Ⱥ�õ���21��2g������____________���ѧʽ����
��3����Ʒ��Na2CO3�����������Ƕ��٣�д��������̣���
��1��д��NaHCO3���ȷֽ�Ļ�ѧ��Ӧ����ʽ_________________________��
��2�����Ⱥ�õ���21��2g������____________���ѧʽ����
��3����Ʒ��Na2CO3�����������Ƕ��٣�д��������̣���
��1��2NaHCO3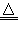 Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O
��2��Na2CO3
��3������Ʒ��Na2CO3��NaHCO3�����ʵ����ֱ�Ϊx��y
Na2CO3 +H2SO4 �� Na2SO4 +CO2��+H2O
2NaHCO3 + H2SO4 =Na2SO4+2CO2��+2H2O
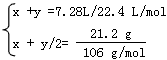
x=0.075mol
��Ʒ��Na2CO3����������Ϊ��0.075mol��106g/mol��28.95g��100%=27.46��
 Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O ��2��Na2CO3
��3������Ʒ��Na2CO3��NaHCO3�����ʵ����ֱ�Ϊx��y
Na2CO3 +H2SO4 �� Na2SO4 +CO2��+H2O
2NaHCO3 + H2SO4 =Na2SO4+2CO2��+2H2O
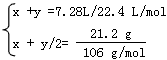
x=0.075mol
��Ʒ��Na2CO3����������Ϊ��0.075mol��106g/mol��28.95g��100%=27.46��

��ϰ��ϵ�д�
�����Ŀ
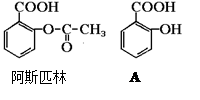
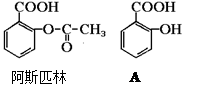
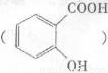 �ɵ��»��߳���ͷʹ�����ĵ�֢״���農��ע��С�մ�(NaHCO3)��Һ������С�մ���ˮ��������е��Ȼ���Ӧ����ˮ�����ƣ�ʹ֢״���⡣д��ˮ������С�մ�Ӧ�Ļ�ѧ����ʽ��
��
�ɵ��»��߳���ͷʹ�����ĵ�֢״���農��ע��С�մ�(NaHCO3)��Һ������С�մ���ˮ��������е��Ȼ���Ӧ����ˮ�����ƣ�ʹ֢״���⡣д��ˮ������С�մ�Ӧ�Ļ�ѧ����ʽ��
��