��Ŀ����
(һ)��1������Ҳ��һ�����ȼ�ϣ�������ȫȼ��ʱ��Ч�ʽ��Ͳ�������ж����������Ⱦ��
��֪�� CH4(g) + 2O2(g) �� CO2(g) + 2H2O(l) ��H1���D890.3 kJ/mol
2CO (g) + O2(g) �� 2CO2(g) ��H2���D566.0 kJ/mol
����鲻��ȫȼ������һ����̼��Һ̬ˮʱ����Ч��ֻ����ȫȼ��ʱ��________��������������1λС������
��2������ȼ�ϵ�ؿ����������������ʡ���ͼ�����ü���ȼ�ϵ�ص��50 mL 2 mol/L���Ȼ�ͭ��Һ��װ��ʾ��ͼ��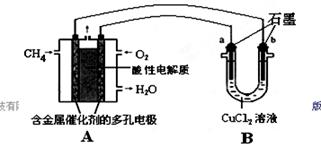
��ش�
�ټ���ȼ�ϵ�صĸ�����Ӧʽ��________��
�ڵ���·����0.1 mol����ͨ��ʱ��________���a����b����������________g��
�������±��Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ�
�ܶȻ�Ksp (25��)��
| ����� | ƽ�ⷽ��ʽ | ƽ�ⳣ��K | Ksp |
| CH3COOH | CH3COOH CH3COO-��H+ CH3COO-��H+ | 1.76��10-5 | |
| H2CO3 | H2CO3 H+��HCO3- H+��HCO3-HCO3-  H+��CO32- H+��CO32- | K1��4.31��10-7 K2��5.61��10-11 | |
| C6H5OH | C6H5OH  C6H5O-��H+ C6H5O-��H+ | 1.1��10-10 | |
| H3PO4 | H3PO4 H+��H2PO4- H+��H2PO4-H2PO4-  H+��HPO32- H+��HPO32-HPO42-  H+��PO43- H+��PO43- | K1��7.52��10-3 K2��6.23��10-8 K3��2.20��10-13 | |
| NH3��H2O | NH3��H2O NH4+��OH- NH4+��OH- | 1.76��10-5 | |
| BaSO4 | BaSO4 Ba2+��SO42- Ba2+��SO42- | | 1.07��10-10 |
| BaCO3 | BaCO3 Ba2+��CO32- Ba2+��CO32- | | 2.58��10-9 |
�ش��������⣺
��1�����ϱ�����������CH3COOH ��HCO3-��C6H5OH ��H2PO4- ���ɿ����ᣬ������������ǿ������˳��Ϊ__________________________(����)��
(2)25��ʱ�����������Ũ�ȵĴ���Ͱ�ˮ��ϣ����Һ�У�c(CH3COO-)______c(NH4+)��(�����������������)
��3��25��ʱ����10ml 0.01mol/L������Һ�еμ�Vml 0.01mol/L��ˮ�������Һ������Ũ�ȹ�ϵ��ȷ����( )��
A�������ҺpH��7����V��10
B�������ҺpH��7����c((NH4+) ��c (C6H5O-) ��c (H+)��c (OH��)
C��V=10ʱ�����Һ��ˮ�ĵ���̶�С��10ml 0.01mol/L������Һ��ˮ�ĵ���̶�
D��V=5ʱ��2c(NH3��H2O)+ 2 c (NH4+)=" c" (C6H5O-)+ c (C6H5OH)
��4������ͼ��ʾ����T1��T2�����¶�������BaSO4��ˮ�еij����ܽ�ƽ�����ߣ��ش��������⣺
����T1�¶�ʱBaSO4�ij����ܽ�ƽ�����ߣ�����˵������ȷ����( )
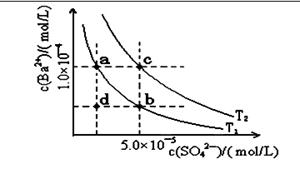
A������Na2SO4��ʹ��Һ��a���Ϊb��
B����T1�����Ϸ�����(��������)����һ��ʱ�� ����BaSO4��������
C�������ܼ�����ʹ��Һ��d���Ϊ������a�� b֮���ijһ��(����a��b)
D�����¿�ʹ��Һ��b���Ϊd��
�� ��1��0.7 ��2�֣� ��2����CH4-8e-+2H2O=CO2+8H+ �� b 3.2 ����2�֣�
�棨1���٢ܢۢ� ��2�֣� ��2�� = ��1�֣� ��3�� D ��2�֣� ��4�� D ��2�֣�
��������������壨1�����鲻��ȫȼ�յ��Ȼ�ѧ����ʽΪ: CH4(g) + O2(g) �� CO(g) + 2H2O(l) ��H���D607.3 kJ/mol,����鲻��ȫȼ������һ����̼��Һ̬ˮʱ����Ч��ֻ����ȫȼ��ʱ��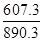 =0.7�� ��2���ٵ����Ϊ���Թʸ�����ӦʽΪ��CH4-8e-+2H2O=CO2+8H+ ��Cu2+�������ŵ缴b����ÿͨ��0.1mol���ӣ���0.5molCu2+�ŵ磬��3.2g �棨1�� KֵԽ������Խǿ ��2��25��ʱ����Ͱ�ˮ�ĵ���̶���ͬ���ʽ��������Ũ�ȵĴ���Ͱ�ˮ��Ϻ���Һ�����ԣ��ʻ��Һ��c(CH3COO-)=c(NH4+)����3����ΪNH3��H2O�ĵ���̶�Զ����C6H5OH������10ml 0.01mol/L������Һ�еμ�Vml 0.01mol/L��ˮ����ˮ���������Ҫ��10mL��Һ��pH>7,A ����B����ѭ����غ㣬����V=10ʱ�����ҺΪ�������Һ�������ˮ��ٽ�ˮ�ĵ��룬�ʻ��Һ��ˮ�ĵ���̶ȴ���10ml 0.01mol/L������Һ��ˮ�ĵ���̶ȣ�C����4�������¶ȣ�BaSO4�ĵ���ƽ�������ƶ���c��SO42-����c(Ba2+)������D����
=0.7�� ��2���ٵ����Ϊ���Թʸ�����ӦʽΪ��CH4-8e-+2H2O=CO2+8H+ ��Cu2+�������ŵ缴b����ÿͨ��0.1mol���ӣ���0.5molCu2+�ŵ磬��3.2g �棨1�� KֵԽ������Խǿ ��2��25��ʱ����Ͱ�ˮ�ĵ���̶���ͬ���ʽ��������Ũ�ȵĴ���Ͱ�ˮ��Ϻ���Һ�����ԣ��ʻ��Һ��c(CH3COO-)=c(NH4+)����3����ΪNH3��H2O�ĵ���̶�Զ����C6H5OH������10ml 0.01mol/L������Һ�еμ�Vml 0.01mol/L��ˮ����ˮ���������Ҫ��10mL��Һ��pH>7,A ����B����ѭ����غ㣬����V=10ʱ�����ҺΪ�������Һ�������ˮ��ٽ�ˮ�ĵ��룬�ʻ��Һ��ˮ�ĵ���̶ȴ���10ml 0.01mol/L������Һ��ˮ�ĵ���̶ȣ�C����4�������¶ȣ�BaSO4�ĵ���ƽ�������ƶ���c��SO42-����c(Ba2+)������D����
���㣺��ѧ��Ӧ�������仯��˵��Һ���ۺϿ���

�±��Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ��ܶȻ�Ksp��25�棩��
| ����� | ���뷽��ʽ | ���볣��K | Ksp |
| H2CO3 | H2CO3 HCO3����H�� HCO3����H��HCO3��  CO32����H�� CO32����H�� | K1��4.31��10��7 K2��5.61��10��11 | �� |
| C6H5OH | C6H5OH C6H5O����H�� C6H5O����H�� | 1.1��10��10 | �� |
| H3PO4 | H3PO4 H2PO4����H�� H2PO4����H��H2PO4��  HPO42����H�� HPO42����H��HPO42��  PO43����H�� PO43����H�� | K1��7.52��10��3 K2��6.23��10��6 K1��2.20��10��13 | �� |
| NH3��H2O | NH3��H2O OH����NH4�� OH����NH4�� | 1.76��10��5 | �� |
| BaSO4 | BaSO4��s�� Ba2����SO42�� Ba2����SO42�� | �� | 1.07��10��10 |
��1��д��C6H5OH��Na3PO4��Ӧ�����ӷ���ʽ��_________________��
��2��25��ʱ����10 mL 0. 01 mol/LC6H5OH��Һ�еμ�V mL 0.1 mol/L��ˮ�������Һ������Ũ�ȹ�ϵ��ȷ����__________������ţ���
a�������ҺpH��7,��V��10
b��V��5ʱ��2c��NH3��H2O����2c��NH4������c��C6H5OH����c��C6H5O����
c��V��10ʱ�����Һ��ˮ�ĵ���̶�С��0.01 mol
 C6H5OH��Һ��ˮ�ĵ���̶�
C6H5OH��Һ��ˮ�ĵ���̶�d�������ҺpH��7����c��NH4������c��C6H5O������c��H������c��OH����
��3��ˮ�ⷴӦ�Ļ�ѧƽ�ⳣ����Ϊˮ�ⳣ������Kb��ʾ������Ȼ�ѧƽ�ⳣ���Ķ��塣25��ʱ��Na2CO3��һ��ˮ�ⷴӦ��ˮ�ⳣ��Kb��____mol/L��
��4����ͼ��ʾ����T1��T2��ͬ�¶�������BaSO4��ˮ�еij����ܽ�ƽ�����ߣ���֪BaSO4��Ksp���¶����߶�����
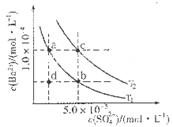
��T2____ 25�棨���������������������
������T1�¶�ʱBaSO4�ij����ܽ�ƽ�����ߣ�����˵����ȷ����____������ţ���
a������Na2SO4����ʹ��Һ��a���Ϊb��
b����T1�����Ϸ����������ߣ�����һ��ʱ������BaSO4��������
c�������ܼ�����ʹ��Һ��d���Ϊ������a��b֮���ijһ�㣨����a��b��
d�����¿�ʹ��Һ��b���Ϊd��
����ȱ�ʾ����ʵ����ǿ��������ȵĶ��壺
�������ѵ���ĵ���ʷ���������Һ��ԭ�е���ʵ��ܷ���������100%��
��֪25��ʱ��������(��)�ĵ����(��ҺŨ�Ⱦ�Ϊ0.1 mol��L��1)���±���
| ��� | ����(��) | ����Ȧ� |
| A | ������Һ(��һ����ȫ����)�� �ڶ��� HSO4- H����SO42- H����SO42- | 10% |
| B | ����������Һ�� HSO4- H����SO42 H����SO42 | 29% |
| C | ��� CH3COOH CH3COO����H�� CH3COO����H�� | 1.33% |
| D | ��� HCl��H����Cl�� | 100% |
��1��25��ʱ��0.1 mol��L��1����������Һ�У�c(H��)�Ӵ�С��˳���� (�����)��
��2��25��ʱ��0.1 mol��L��1������Һ��HSO4-�ĵ����С����ͬ�¶���0.1 mol��L��1��������
��Һ��HSO4-�ĵ���ȣ���ԭ���� ��
��3������ĵ���ƽ�ⳣ��K�ı���ʽ�� ������ĵ���ƽ�ⳣ��
K�����Ȧ��Ĺ�ϵʽΪ��K= ���ú����Ĵ���ʽ��ʾ��
���仯�����й㷺��Ӧ�ã���SO2���ʵ��о��Ǹ��л�ѧ��ѧ��һ����Ҫ���ݡ�
I���Ա��о���һ����Ҫ���о�������������ĵ��ʼ����ֻ����ﰴ���±���ʾ�ֳ�3 �飬���2��������M�Ļ�ѧʽ�� ��
| ��1�� | ��2�� | ��3�� |
| S (����) | SO2��H2SO3��M��NaHSO3 | SO3��H2SO4��Na2SO4��NaHSO4 |

��1������ʵ�鷽������������ʵ������ȡ����SO2���� ��
A��Na2SO3��Һ��HNO3 B��Na2SO3����Ũ����
C���������ڴ�����ȼ�� D��ͭ���ȵ�Ũ����
��2��װ��C�������dz�ȥ�����SO2����ֹ��Ⱦ��������֪��������������Һ����SO2�� �����У������õ�Na2SO3��NaHSO3�Ļ����Һ�������£���ҺpH��n(SO32��)��n(HSO3��)�仯��ϵ���±�
| n(SO32��)��n(HSO3��) | 91��9 | 1��1 | 9��91 |
| pH | 8.2 | 7.2 | 6.2 |
������Һ��n(SO32��)��n(HSO3��) =10��1ʱ����Һ������Ũ�ȹ�ϵ��ȷ���� ��
A��c(Na+)+ c(H+)= 2c(SO32��)+ c(HSO3��)+ c(OH��)
B��c(Na+)��c(HSO3��)��c(SO32��)��c(OH��)��c(H+)
C��c(Na+)��c(SO32��)��c(HSO3��)��c(OH��)��c(H+)
��3��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ȡA�з�Ӧ�����Һ�ֳ����ݣ������������̽��ʵ�飬�������۲��������ǵ�̽�����̣���ѡ�Լ���KMnO4��Һ��KSCN��Һ��BaCl2��Һ��ϡ���ᡢϡ���ᡢϡ�� �ᡢBa(NO3)2��Һ�����Ƶ���ˮ��
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| ������ | ����һ����Һ�м���KMnO4��Һ��Һ | �Ϻ�ɫ��ȥ | SO2��Fe3+��Ӧ������Fe2+ |
| ������ | ���ڶ�����Һ�м��� | | SO2��Fe3+��Ӧ������Fe2+ |
| ������ | ���ڶ�����Һ�м��� | | SO2��Fe3+��Ӧ������SO42�� |
���������ٵó��Ľ����Ƿ���� ��ԭ�� ��
���������Ƶķ������뷽���۾��������ҵõ���Ӧ���ۣ����㽫�������������
��4��װ��B���ܱ���Br���Ļ�ԭ������SO2�������� ��
�ҹ�ij���͵��ͭ������ҵ����ұ��������ͭ��������ʴﵽ97����87������ͼ��ʾ��ұ���ӹ������̣�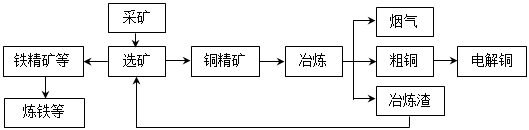
ұ���е���Ҫ��Ӧ��Cu2S + O2 =" 2Cu" + SO2
��1�������е���Ҫ������________________���������Դ�����ʺͼ��ſ��ǣ����ۺ����÷�ʽ����___________��
��2����ⷨ��ͭʱ��������____________�����ͭ�塱��ͭ�塱������ͭ�к��еĽ����Ե��ʵ���ʽ�ڵ���_______________������������������IJ۵ף������ĵ缫��Ӧʽ��_________________________________________��
��3���ھ���ͭ�Ĺ����У��������Һ��c(Fe2+)��c(Zn2+)���������Ӱ���һ����⡣
�������ʵ��ܶȻ�������KSP����
| ���� | Fe(OH)2 | Fe(OH)3 | Zn(OH)2 | Cu(OH)2 |
| KSP | 8.0��10��16 | 4.0��10��38 | 3.0��10��17 | 2.2��10��20 |
���ڵ��Һ��pH�dz�ȥ�������ӵij��÷����������ϱ����ܶȻ������жϣ����е����ʵ���Ũ�ȵ�Fe2+��Zn2+��Fe3+��Cu2+����Һ����pH�������ȳ���������������______________��
һ�ַ������ȼ��������H2O2���ٵ���pH��4���ҡ�����H2O2������Ӧ�����ӷ���ʽΪ___________________________________________________________________________��
 2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
2Ca2����2K����Mg2����4SO42-��2H2O��Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�

 H+ + H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3��
H+ + H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3��