��Ŀ����
�����Ǹ������Դ���⣬��ˮ��Դ�����þ��зdz������ķ�չǰ������ˮ����Ԫ����Br����ʽ���ڣ���ҵ���ÿ����������Ӻ�ˮ����ȡ��Ĺ�������������ʾ��
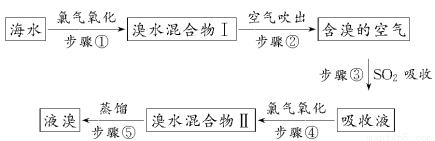
(1)��������Ӧ�����ӷ���ʽΪ_______________________________��
(2)��������Ӧ�Ļ�ѧ����ʽΪ_________________________________��
(3)Br��ԭ��������________�������ڱ���λ�ڵ�________���ڡ�________�塣
(4)����������Ĺ����У��¶�Ӧ������80��90�����¶ȹ�����Ͷ������������������ԭ��________________��
(5)Ϊʲô��ֱ��������ˮ�����������Ҫ������ˮ����������������ó�Һ�壿
(1)2Br����Cl2=Br2��2Cl����(2)SO2��Br2��2H2O=2HBr��H2SO4��(3)35���ġ���A��(4)�¶ȹ��ߣ�����ˮ�������������ų�����������ˮ���ӣ��¶ȹ��ͣ��岻����ȫ�����������ʽ��͡�(5)����ˮ�������������Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ�����������������ɱ��ߣ�����������������SO2���ա������������Ȳ���ʵ�����ǽ���ˮŨ����
��������(1)Cl�ķǽ����Դ���Br����ˮ�к��е�Br���������û���Cl2��2Br��=Br2��2Cl����
(2)�����е�Br2��SO2��Ӧ���õ�����Ũ��Br������Һ��SO2��2H2O��Br2=2HBr��H2SO4��
(3)��Ϊ�������ڣ���A��Ԫ�أ����ݸ�����Ԫ�صĸ����������������ϡ������Ԫ�ص�ԭ���������ɴ��Ƴ�Br��ԭ��������2��8��8��18��1��35��
(4)�����������ԭ�����лش�ˮ�ķе�Ϊ100�����¶ȹ���ˮ�����������¶ȹ��ͣ���Ļӷ������٣�������ȫ�����������ʽ��͡�
(5)��ˮ��Br���ĺ����ܵͣ�������� ˮ�������������ĺ����ܵͣ�ֱ��������Һ������ܴ��ܺĸߣ��ɱ����ӣ�����һϵ�д����������ˮ�����������Br2��Ũ����������ʱ�����账����Һ���������٣��ܺĽ��ͣ��ɱ����͡�

 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�