��Ŀ����
Ŀǰ�ⶨ������SO2������Ҫ��������ԭ��Ӧ����֪SO2����������KMnO4��Һ��Ӧʱ��MnO4-����ԭΪMn2����SO2��������SO42-��Ϊ�ⶨij�ط��Ŀ�����SO2�Ϳ���������ĺ�������ͬѧ���������ͼ��ʾ��ʵ��װ�ã�

������ ��
�� ��ʾ�ܱ�������
��ʾ�ܱ������� ��ʾ�������ٹ�(��λʱ����ͨ�����������㶨����������������������)��
��ʾ�������ٹ�(��λʱ����ͨ�����������㶨����������������������)�� ��ʾ���������������������տ�����Ŀ������D����ʾ���������
��ʾ���������������������տ�����Ŀ������D����ʾ���������
������KMnO4��Һ���Ϊ200 mL��Ũ��Ϊ0.1 mol��L��1��
�ش��������⣺
(1)����200 mL 0.1 mol��L��1����KMnO4��Һ�����õ���������ʹ�õ��Ⱥ�˳��������________��________��________����������________��________����������������
____________________________________________________________��
(2)д������ƽ�ⶨSO2���������ӷ���ʽ��_______________________________________��
(3)ʵ��������������_____________________________________________________��
��Ҫ�ⶨ�����п���������ĺ���(g��L��1)������Ҫ�����������________��
(4)��ͬѧ����ͬ���ķ�������������SO2�ĺ���������õ���ֵ���DZ�ʵ�ʺ���ƫ�ͣ�����ܵ�ԭ����(������Һ���ơ���������ȡ�����ֶ���������)��____________________________________________________��

������
 ��
�� ��ʾ�ܱ�������
��ʾ�ܱ������� ��ʾ�������ٹ�(��λʱ����ͨ�����������㶨����������������������)��
��ʾ�������ٹ�(��λʱ����ͨ�����������㶨����������������������)�� ��ʾ���������������������տ�����Ŀ������D����ʾ���������
��ʾ���������������������տ�����Ŀ������D����ʾ���������������KMnO4��Һ���Ϊ200 mL��Ũ��Ϊ0.1 mol��L��1��
�ش��������⣺
(1)����200 mL 0.1 mol��L��1����KMnO4��Һ�����õ���������ʹ�õ��Ⱥ�˳��������________��________��________����������________��________����������������
____________________________________________________________��
(2)д������ƽ�ⶨSO2���������ӷ���ʽ��_______________________________________��
(3)ʵ��������������_____________________________________________________��
��Ҫ�ⶨ�����п���������ĺ���(g��L��1)������Ҫ�����������________��
(4)��ͬѧ����ͬ���ķ�������������SO2�ĺ���������õ���ֵ���DZ�ʵ�ʺ���ƫ�ͣ�����ܵ�ԭ����(������Һ���ơ���������ȡ�����ֶ���������)��____________________________________________________��
(1)������ƽ��ҩ�ס��ձ���200 mL����ƿ����ͷ�ιܡ����������
(2)5SO2��2MnO4-��2H2O=5SO42-��2Mn2����4H��
(3)��ֹ������������������ܻ�������Ը��������Һ�У�ʹ����������������ǰ�����������������ʢ��������������(��װ���ñ�ű�ʾ�ش𣬻�����������������ʢ��������������������ֵ)
(4)ͨ���������ʹ��죬��������δ�����Ը��������Һ��ַ�Ӧ���Ѿ����ų�
(2)5SO2��2MnO4-��2H2O=5SO42-��2Mn2����4H��
(3)��ֹ������������������ܻ�������Ը��������Һ�У�ʹ����������������ǰ�����������������ʢ��������������(��װ���ñ�ű�ʾ�ش𣬻�����������������ʢ��������������������ֵ)
(4)ͨ���������ʹ��죬��������δ�����Ը��������Һ��ַ�Ӧ���Ѿ����ų�
(1)����200 mL 0.1 mol��L��1����KMnO4��Һ�����ù�����������һ��Ũ�ȵ���Һ������������ƽ��ҩ�ס��ձ���200 mL����ƿ����ͷ�ιܵȣ���������Һ�����в��������ܽ���������ڽ�������ܽ⣬ת����Һʱ���������á�
(2)SO2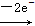 SO42-��MnO4-
SO42-��MnO4-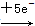 Mn2�����ɵ����غ㡢����غ��Ԫ���غ�Ĵ�����ƽ����ʽ��
Mn2�����ɵ����غ㡢����غ��Ԫ���غ�Ĵ�����ƽ����ʽ��
(2)SO2
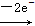 SO42-��MnO4-
SO42-��MnO4-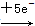 Mn2�����ɵ����غ㡢����غ��Ԫ���غ�Ĵ�����ƽ����ʽ��
Mn2�����ɵ����غ㡢����غ��Ԫ���غ�Ĵ�����ƽ����ʽ��
��ϰ��ϵ�д�
�����Ŀ

 ����
���� �����ն�
�����ն� ����
����