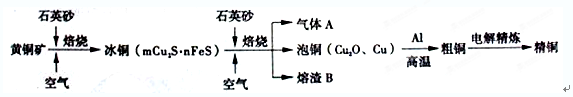
��Ŀ����
�Ӻ�ˮ��ȡþ������������£���ش�������⡣
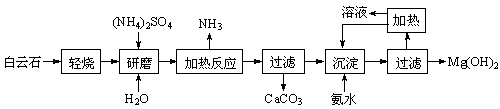
��1���Ӻ�ˮ����ȡþ����������ͼ��ʾ����ͼ������Ҫ�����
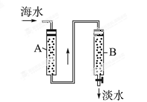
i.��ˮ���������ǰ���Ժ�ˮ���д������������ַ�����
����һ����ɹ�κ��±ˮͨ������أ�
������������������Ũ����ĺ�ˮͨ������ء�
��������________��������������_________________________________________��
ii.��Ӧ�ٵ����ӷ�����________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_______________________________________________��
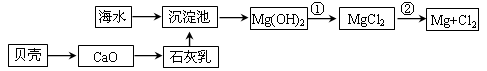
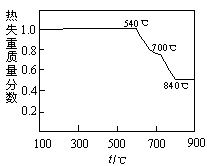
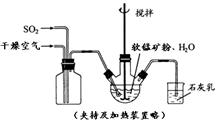
��2���Ӻ�ˮ����ȡ�����������ͼ��ʾ����ͼ������Ҫ�����
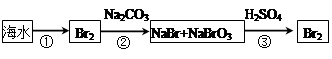

i.���̢��У�������Լ���___________��
ii.���̢��У�����Һ�д����ȿ��������崵�����ô������գ������ȿ�����Ŀ����______________________________________________________________________��
iii.���̢��з�Ӧ�Ļ�ѧ����ʽ��____________________________________________��
iv.�����յõ����嵥������Ȼ����������Cl2�����ȥ�����ʵķ�����__________________________________________________��������ӷ���ʽ�ش𣩡�
��1���Ӻ�ˮ����ȡþ����������ͼ��ʾ����ͼ������Ҫ�����

i.��ˮ���������ǰ���Ժ�ˮ���д������������ַ�����
����һ����ɹ�κ��±ˮͨ������أ�
������������������Ũ����ĺ�ˮͨ������ء�
��������________��������������_________________________________________��
ii.��Ӧ�ٵ����ӷ�����________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_______________________________________________��
��2���Ӻ�ˮ����ȡ�����������ͼ��ʾ����ͼ������Ҫ�����

i.���̢��У�������Լ���___________��
ii.���̢��У�����Һ�д����ȿ��������崵�����ô������գ������ȿ�����Ŀ����______________________________________________________________________��
iii.���̢��з�Ӧ�Ļ�ѧ����ʽ��____________________________________________��
iv.�����յõ����嵥������Ȼ����������Cl2�����ȥ�����ʵķ�����__________________________________________________��������ӷ���ʽ�ش𣩡�
��9�֣���1��i. һ Ҫ���Ⱥ�ˮ���������Դ���˷�����Դ
ii. Mg(OH)2��2H����Mg2����2H2O �� MgCl2(����) Mg��Cl2��
Mg��Cl2��
��2��i. Cl2
ii.�¶����ߣ�������ܽ�ȼ�С��������������巢����Ӧ�����Խ���Һ�壬���������������ݳ�
iii.5NaBr��NaBrO3��3H2SO4��3Br2��3Na2SO4��3H2O��2�֣�
iv.���������廯�ƣ������л��ܼ���������ᴿ��Cl2��2Br����Br2��2Cl��
ii. Mg(OH)2��2H����Mg2����2H2O �� MgCl2(����)
 Mg��Cl2��
Mg��Cl2����2��i. Cl2
ii.�¶����ߣ�������ܽ�ȼ�С��������������巢����Ӧ�����Խ���Һ�壬���������������ݳ�
iii.5NaBr��NaBrO3��3H2SO4��3Br2��3Na2SO4��3H2O��2�֣�
iv.���������廯�ƣ������л��ܼ���������ᴿ��Cl2��2Br����Br2��2Cl��
�����������1��i.��������Ũ����ˮ��Ҫ���Ⱥ�ˮ���Ӷ����Ĵ�������Դ���˷�����Դ�����Է���һ��á�
ii.������þת��Ϊ�Ȼ�þ����Ҫ��������þ�ܽ��������У���Ӧ�����ӷ���ʽ��Mg(OH)2��2H����Mg2����2H2O��þ�ǻ��õĽ�������Ҫ��ⷨұ����������ڵ��Ȼ�þ�������ɽ���þ����������Ӧ�Ļ�ѧ����ʽ��MgCl2(����)
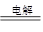 Mg��Cl2����
Mg��Cl2������2��i.��ˮ�е������������ӵ��γɴ��ڵģ���Ҫ���������������������ɵ����壬��˹��̢��У�������Լ���Cl2��
ii.�����¶����ߣ�������ܽ�ȼ�С��������������巢����Ӧ�����Խ���Һ�壬���������������ݳ������Ҫ�����ȿ�����
iii.�����������£��������ܺ��廯�Ʒ���������ԭ��Ӧ���ɵ����壬��Ӧ�Ļ�ѧ����ʽ��5NaBr��NaBrO3��3H2SO4=3Br2��3Na2SO4��3H2O��
iv. ��������ǿ�����ԣ�����Ҫ��ȥ�������е��������������Լ��������廯�ƣ������л��ܼ���������ᴿ����Ӧ�����ӷ���ʽ��Cl2��2Br��=Br2��2Cl����
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����Ӧ�������������������������Ժ�ˮ���ۺ�Ӧ��Ϊ���壬�����ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧϰ��֪����

��ϰ��ϵ�д�
�����Ŀ
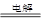 2Na��Cl2��
2Na��Cl2��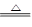 Mg��H2O
Mg��H2O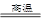 2Fe��3CO2
2Fe��3CO2