��Ŀ����
������X��Y��Z���ɶ����ڵ�����Ԫ��������϶��ɡ�X��Y��Z֮���ת����ϵ����ͼ��ʾ(�ޱ�ʡ�Ե�����)����֪��X��N��W�����¾�ΪҺ̬���ʣ���X��N�����Ԫ����ͬ��X��Z������ͬ����Է�������������������Ϣ��������������⣺
(1)����ת����ϵͼ���漰�Ļ�����Ӧ���������������������������� ��
(2)X��Y��Z��W�Ļ�ѧʽ�����ǣ�X_________��Y_________��Z_________��W_________��
(3)д��X��Y��X��Z��Ӧ�Ļ�ѧ����ʽ��
X+Y��_______________________________________________��
X+Z��_______________________________________________��

(1)���Ϸ�Ӧ ��1�֣�
(2)H2O2 SO2 H2S H2SO4 ��ÿ�վ�Ϊ1�֣�
(3)H2O2+SO2=H2SO4 ��2�֣� H2O2+H2S=S��+2H2O��2�֣�
����ʽ�з�Ӧ������������Ϊ0�֣���ƽ��Ϊ0�֣��������������δ���1�֡�

��ϰ��ϵ�д�
�����Ŀ
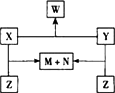
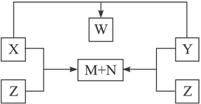

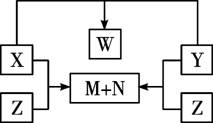 ������X��Y��Z���ɶ����ڵ�����Ԫ��������϶��ɣ�X��Y��Z֮���ת����ϵ����ͼ��ʾ���ޱ�ʡ�Ե����ʣ���
������X��Y��Z���ɶ����ڵ�����Ԫ��������϶��ɣ�X��Y��Z֮���ת����ϵ����ͼ��ʾ���ޱ�ʡ�Ե����ʣ���