��Ŀ����
�������ƣ�NaClO2����һ����Ҫ������������Ҫ����ˮ��ɰ�ǡ���֬��Ư����ɱ������������ȡ�������ƵĹ������̣�
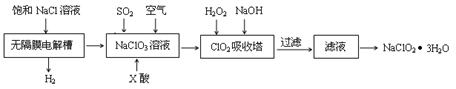
��֪����NaClO2���ܽ�������¶����߶������ʵ������¿ɽᾧ������
��ClO2����ֻ�ܱ�����ϡ��״̬���Է�ֹ��ը�Էֽ⣬�����ֺϳ����á�
��ClO2���������Ժͼ�����Һ�в����ȶ����ڡ�
(1)����Ĥ�����г������һ��ʱ�������������NaClO3����д�������ĵ缫��Ӧ����ʽ�� ��
(2)��Ӧ����ClO2������ҪX���ữ��ԭ��Ϊ�� ��X��Ϊ ��
(3)�������ڵ��¶Ȳ��ܹ��ߵ�ԭ��Ϊ��
(4)��������ClO2���Ż�ԭ���IJ�ͬ����Һ����Եı仯�ɱ���ԭΪClO2����Cl����ClO2��S2����ԭΪClO2����Cl����ת��������ҺpH�Ĺ�ϵ����ͼ��ʾ��
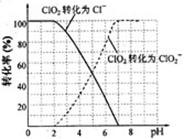
��д��pH��2ʱClO2��S2����Ӧ�����ӷ���ʽ�� ��
(5)�ڶ�����NaClO3��Һ��ͨSO2��ͬʱͨ�������ԭ��Ϊ�� ��
(6)����Һ�еõ�NaClO2��3H2O�־���IJ�������Ϊ
�� �� ��

��֪����NaClO2���ܽ�������¶����߶������ʵ������¿ɽᾧ������
��ClO2����ֻ�ܱ�����ϡ��״̬���Է�ֹ��ը�Էֽ⣬�����ֺϳ����á�
��ClO2���������Ժͼ�����Һ�в����ȶ����ڡ�
(1)����Ĥ�����г������һ��ʱ�������������NaClO3����д�������ĵ缫��Ӧ����ʽ�� ��
(2)��Ӧ����ClO2������ҪX���ữ��ԭ��Ϊ�� ��X��Ϊ ��
(3)�������ڵ��¶Ȳ��ܹ��ߵ�ԭ��Ϊ��
(4)��������ClO2���Ż�ԭ���IJ�ͬ����Һ����Եı仯�ɱ���ԭΪClO2����Cl����ClO2��S2����ԭΪClO2����Cl����ת��������ҺpH�Ĺ�ϵ����ͼ��ʾ��

��д��pH��2ʱClO2��S2����Ӧ�����ӷ���ʽ�� ��
(5)�ڶ�����NaClO3��Һ��ͨSO2��ͬʱͨ�������ԭ��Ϊ�� ��
(6)����Һ�еõ�NaClO2��3H2O�־���IJ�������Ϊ
�� �� ��
��12�֣�(1) Cl��+6e��+6OH����ClO3��+3H2O (2) ClO2ֻ�������Ի����д��ڣ�����
(3)��ֹH2O2�ֽ� (4) 2ClO2+5S2��+ 8H+ ��2Cl��+5S��+4H2O
(5)ϡ��ClO2���壬��ֹ��ը (6)����Ũ������ȴ�ᾧ������
(3)��ֹH2O2�ֽ� (4) 2ClO2+5S2��+ 8H+ ��2Cl��+5S��+4H2O
(5)ϡ��ClO2���壬��ֹ��ը (6)����Ũ������ȴ�ᾧ������
�����������1������������ʧȥ���ӣ�������Һ�е�������������ʧȥ���ӣ���˷�Ӧ�ĵ缫��Ӧʽ��Cl��+6e��+6OH����ClO3��+3H2O��
��2������ClO2���������Ժͼ�����Һ�в����ȶ����ڣ�����ֻ�������Ի����д��ڡ����������������£��������������ᷢ��������ԭ��Ӧ������XӦ�������ᡣ
��3��H2O2���ȶ����¶ȹ��ߣ�H2O2���ֽ⣬���������¶Ȳ��ܹ��ߣ���Ŀ���Ƿ�ֹH2O2�ֽ⡣
��4������ͼ���֪��pH��2ʱClO2����ԭΪCl�������Ը÷�Ӧ�����ӷ���ʽ��2ClO2+5S2��+ 8H+ ��2Cl��+5S��+4H2O��
��5������Ϣ�ڿ�֪����ClO2�ֽⱬը��һ����ϡ����������ϡ�ͣ�Ŀ����ϡ��ClO2���壬��ֹ��ը��
��6������Һ�еõ����ᾧˮ�ľ��壬ֻ�ܲ�ȡ������Ũ������ȴ�ᾧ������ͨ�����˵õ��־��壬���Դ���Һ�еõ�NaClO2·3H2O�־���IJ�������Ϊ����Ũ������ȴ�ᾧ�����ˡ�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ�����������������ͷ�����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������淶�Ͻ���ʵ�����������������ѧ���Ƚ�İ�������������Ʊ�Ϊ�������Թ������ⷨ�Ʊ���������Ϊ���ߣ�����ѧ���Ķ���Ŀ��ȡ��Ϣ����������Ũ�ȸ�������⡢��������ԭ��Ӧ���֪ʶ�����á��й�ʵ������ͼ�ʵ��������������Լ������龳���ۺ�����֪ʶ����������������Ŀ��һ�����Ѷȡ�

��ϰ��ϵ�д�
�����Ŀ
 Mn2+ +Cl2�� + 2H2O
Mn2+ +Cl2�� + 2H2O Cl2 ��+H2 ��+2OH��
Cl2 ��+H2 ��+2OH�� Cl2�� �� Mn2+ ��H2O
Cl2�� �� Mn2+ ��H2O C
C
