��Ŀ����
����Ŀ���ִ���ѧ�������������о���Һ�����ӷ�Ӧ����Ҫ�ֶΣ��絼���Ǻ����������Һ����������С��������������Һ�絼�ʱ仯����ȷ���ζ���Ӧ���յ㡣��ͼ��ijͬѧ��0.1 mol��L��1KOH��Һ�ζ������Ϊ20 mL��Ũ�Ⱦ�Ϊ0.1 mol��L��1��HCl��Һ��CH3COOH��Һ��������Һ�絼�ʱ仯ʾ��ͼ��
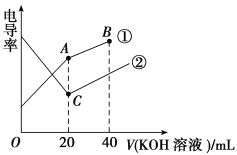
(1)����������0.1 mol��L��1KOH��Һ�ζ�________��Һ�ĵζ����ߡ�
(2)A����Һ��pH________(�����������������)C����Һ��pH��
(3)B�����Һ������Ũ�ȴ�С˳����___________________________________��
(4)����������Һ�絼�ʱ仯ʾ��ͼ����������ȷ��ʾ��NH3��H2O��Һ�ζ�HCl��CH3COOH�����Һ�ĵζ����ߵ���________(����ĸ)��
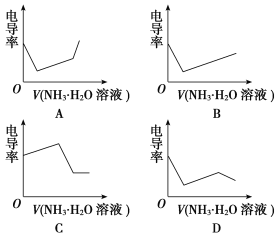
���𰸡�(1)HCl (2)�� (3)c(K��)��c(OH��)��c(CH3COO��)��c(H��) (4)D
��������(1)��Һ��������������Ũ�ȳ����ȣ�CH3COOH��������ʣ���Һ������Ũ�Ƚ�С������KOH��Һ����Һ������Ũ��������Һ��������ǿ��HCl��ǿ����ʣ�����KOH��Һ���룬��Һ�����������Һ������Ũ�ȼ�С����Һ������������������ȫ��Ӧʱ����Ũ����С����������KOH��Һ������Ũ����������Һ����������ǿ������ͼ֪������������0.1 mol��L��1KOH��Һ�ζ�HCl��Һ�ĵζ����ߣ�����������0.1 mol��L��1KOH��Һ�ζ�CH3COOH��Һ�ĵζ����ߡ�(2)A����Һ������ΪCH3COOK��Ϊǿ�������Σ�ˮ���Լ��ԣ���C����Һ��������KCl��Ϊǿ��ǿ���Σ���Һ�����ԡ�(3)B��ʱ�������Һ�к������ʵ���Ũ�ȵ�KOH��CH3COOK����Һ�ʼ��ԣ�CH3COO��ˮ��̶Ƚ�С����������غ������Ũ�ȴ�С˳����c(K��)��c(OH��)��c(CH3COO��)��c(H��)��(4)�Ȼ�����ǿ����ʣ�CH3COOH��������ʣ��μӵ���������Ⱥ��Ȼ��ⷴӦ����ǿ������Ȼ�泥�����Һ�������������Һ��ϡ�ͣ����Ե絼���½������Ȼ�����ȫ���кͺ�һˮ�ϰ��������������CH3COOH��Ӧ����ǿ����ʴ���泥����Ե絼������CH3COOH��ȫ��Ӧ�����μӰ�ˮ����Һ��ϡ�ͣ��絼�����½����ơ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������ʵ�鲻�ܴﵽʵ��Ŀ�ĵ���(����)
��� | ʵ����� | ʵ��Ŀ�� |
A | Cl2��Br2�ֱ���H2��Ӧ | �Ƚ��ȡ���ķǽ�����ǿ�� |
B | ��MgCl2��AlCl3��Һ�зֱ�ͨ��NH3 | �Ƚ�þ�����Ľ�����ǿ�� |
C | �ⶨ��ͬ���ʵ���Ũ�ȵ�Na2CO3��Na2SO4��Һ��pH | �Ƚ�̼����ķǽ�����ǿ�� |
D | Fe��Cu�ֱ���ϡ���ᷴӦ | �Ƚ�����ͭ�Ľ�����ǿ�� |
A. A B. B C. C D. D