��Ŀ����
 ��֪��
��֪����Ũ���������ˮ�ԣ��ڼ���������Ҳ����̼��ͭ�ȵ��ʷ�Ӧ��
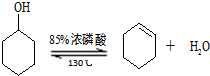
�����ɻ���ϩ�ķ�Ӧ��

����Ӧ���ʲ�������
| �л��� | �ܶ�/ g?cm-3 |
�۵�/ �� |
�е�/ �� |
�ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
������Ʊ���Ʒ
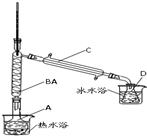
��1����12.5mL������������ͼ��Ӧװ���е�Բ����ƿA�ڣ��ټ���2mL��������Ϊ85%��Ũ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ������ƿD�ڵõ�����ϩ��Ʒ����������B��������
��������
��������
���˷�Ӧ�в���Ũ�����ԭ������ֹ����̼��������
��ֹ����̼��������
����2����Ӧװ�õ�ˮԡ�¶ȿ�����85�����ң�������130�棬����Ϊ
�ӽ�����ϩ�ķе㣬��ֹ��Ӧ�¶ȹ��߶�̼��������ǡ���ķ�Ӧ����
�ӽ�����ϩ�ķе㣬��ֹ��Ӧ�¶ȹ��߶�̼��������ǡ���ķ�Ӧ����
��Ե�ʣ�������Ʊ���Ʒ
��3��Ϊ���ᴿ����ϩ����ȥ��Ʒ�еĻ��������������ˮ�����ʣ��������²�����
�ټ��뱥��ʳ��ˮ���������á��ֲ㡢��Һ������ʳ�ο���
����
����
���ã��ڷ�Һ������
C
C
������ţ��Լ�ϴ�ӣ�A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
�������Ʒ�м�����ˮ�Ȼ��ƺ���������ˮ�Ȼ���Ŀ���ǣ�
����ˮ��
����ˮ��
����4���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ������������ǣ�
BC
BC
������ţ���A�������Ը��������Һ B���ý����� C���ⶨ�е㣮
��������1���ӱ��п���������ϩ���ӷ�����ȼ�����ʣ����ԣ����ռ�ʱҪ��ȴ��Һ��-���ɣ�
��2���ռ�ʱ��ֻҪҺ���¶ȴﵽ����ϩ�ķе㣬�ܰ�����������Ϳ����ˣ��¶�̫���������¶�̫�ߣ����ռ��������ѣ�Ҳ���������ʺ�����
��3���ٻ���ϩ�����࣬�������Ȼ�����Һ�����ܶȱ�ˮС�������á��ֲ��ϩ���ϲ㣬
�ڷ�Һ��ϩ��Ʒ�л�������������ͻ��������������ṩ���ʵ����ʷ�����
����ˮ�Ȼ��ƾ�����ˮ���ã�
��4�����ݻ����û�й̶��ķе㣬���������й̶��ķе㣬ͨ���ⶨ����ϩ��Ʒ�ͻ���ϩ��Ʒ�ķе㣬���жϲ�Ʒ�Ĵ��ȣ�
��2���ռ�ʱ��ֻҪҺ���¶ȴﵽ����ϩ�ķе㣬�ܰ�����������Ϳ����ˣ��¶�̫���������¶�̫�ߣ����ռ��������ѣ�Ҳ���������ʺ�����
��3���ٻ���ϩ�����࣬�������Ȼ�����Һ�����ܶȱ�ˮС�������á��ֲ��ϩ���ϲ㣬
�ڷ�Һ��ϩ��Ʒ�л�������������ͻ��������������ṩ���ʵ����ʷ�����
����ˮ�Ȼ��ƾ�����ˮ���ã�
��4�����ݻ����û�й̶��ķе㣬���������й̶��ķе㣬ͨ���ⶨ����ϩ��Ʒ�ͻ���ϩ��Ʒ�ķе㣬���жϲ�Ʒ�Ĵ��ȣ�
����⣺��1���������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ��������Ũ���������ˮ�ԣ����´�������̼�����ʴ�Ϊ��������������ֹ����̼����������
��2���ռ�ʱ��ֻҪҺ���¶ȴﵽ����ϩ�ķе㣬�ܰ�����������Ϳ����ˣ��¶�̫���������¶�̫�ߣ����·�Ӧ�¶ȹ��߶�̼����
�ʴ�Ϊ���ӽ�����ϩ�ķе㣬��ֹ��Ӧ�¶ȹ��߶�̼��������ǡ���ķ�Ӧ���ʣ�
��3���ٻ���ϩ�����࣬�������Ȼ�����Һ���������������ã��ʴ�Ϊ��������
�����ڷ�Һ��ϩ��Ʒ�л�������������ͻ������������Ʊ����������ᴿ����ʱ��c��Na2CO3��Һ��ϴ�ӿɳ�ȥ�ᣬ���������Ը�����أ��������������ϩ���ʴ�Ϊ��C��
��ˮ�Ȼ��ƾ�����ˮ���ã��ʴ�Ϊ������ˮ�֣�
��4�������Ʒ�뾫Ʒ�ɼ�������ƣ��۲��Ƿ�������������������壬���Ǿ�Ʒ�������û�й̶��ķе㣬���������й̶��ķе㣬ͨ���ⶨ����ϩ��Ʒ�ͻ���ϩ��Ʒ�ķе㣬���жϲ�Ʒ�Ĵ��ȣ��ʴ�Ϊ��BC��
��2���ռ�ʱ��ֻҪҺ���¶ȴﵽ����ϩ�ķе㣬�ܰ�����������Ϳ����ˣ��¶�̫���������¶�̫�ߣ����·�Ӧ�¶ȹ��߶�̼����
�ʴ�Ϊ���ӽ�����ϩ�ķе㣬��ֹ��Ӧ�¶ȹ��߶�̼��������ǡ���ķ�Ӧ���ʣ�
��3���ٻ���ϩ�����࣬�������Ȼ�����Һ���������������ã��ʴ�Ϊ��������
�����ڷ�Һ��ϩ��Ʒ�л�������������ͻ������������Ʊ����������ᴿ����ʱ��c��Na2CO3��Һ��ϴ�ӿɳ�ȥ�ᣬ���������Ը�����أ��������������ϩ���ʴ�Ϊ��C��
��ˮ�Ȼ��ƾ�����ˮ���ã��ʴ�Ϊ������ˮ�֣�
��4�������Ʒ�뾫Ʒ�ɼ�������ƣ��۲��Ƿ�������������������壬���Ǿ�Ʒ�������û�й̶��ķе㣬���������й̶��ķе㣬ͨ���ⶨ����ϩ��Ʒ�ͻ���ϩ��Ʒ�ķе㣬���жϲ�Ʒ�Ĵ��ȣ��ʴ�Ϊ��BC��
�������������л��ϳ�Ϊ�����ۺϿ�����ʵ�����ƻ���ϩ��֪ʶ�ʹ����������ʣ�������ͬѧ���������ʵ�����������Ŀ�Ѷ��еȣ�ע�����ʵ��ԭ���ͷ������ر���ʵ��Ļ���������ѧϰ��ע����ۣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��֪��
��Ũ���������ˮ�ԣ��ڼ���������Ҳ����̼��ͭ�ȵ��ʷ�Ӧ��
�����ɻ���ϩ�ķ�Ӧ��
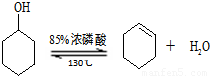
����Ӧ���ʲ�������
ij��ѧС������ͼ��ʾװ���Ի�����Ϊԭ���Ʊ�����ϩ���䲽�����£�
������Ʊ���Ʒ
��1����12.5mL������������ͼ��Ӧװ���е�Բ����ƿA�ڣ��ټ���2mL��������Ϊ85%��Ũ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ������ƿD�ڵõ�����ϩ��Ʒ����������B��������______���˷�Ӧ�в���Ũ�����ԭ����______��
��2����Ӧװ�õ�ˮԡ�¶ȿ�����85�����ң�������130�棬����Ϊ______��Ե�ʣ�
������Ʊ���Ʒ
��3��Ϊ���ᴿ����ϩ����ȥ��Ʒ�еĻ��������������ˮ�����ʣ��������²�����
�ټ��뱥��ʳ��ˮ���������á��ֲ㡢��Һ������ʳ�ο���______���ã�
�ڷ�Һ������______������ţ��Լ�ϴ�ӣ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
�������Ʒ�м�����ˮ�Ȼ��ƺ���������ˮ�Ȼ���Ŀ���ǣ�______��
��4���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ������������ǣ�______������ţ���
A�������Ը��������Һ B���ý����� C���ⶨ�е㣮
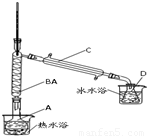
��Ũ���������ˮ�ԣ��ڼ���������Ҳ����̼��ͭ�ȵ��ʷ�Ӧ��
�����ɻ���ϩ�ķ�Ӧ��

����Ӧ���ʲ�������
| �л��� | �ܶ�/ g?cm-3 | �۵�/ �� | �е�/ �� | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
������Ʊ���Ʒ
��1����12.5mL������������ͼ��Ӧװ���е�Բ����ƿA�ڣ��ټ���2mL��������Ϊ85%��Ũ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ������ƿD�ڵõ�����ϩ��Ʒ����������B��������______���˷�Ӧ�в���Ũ�����ԭ����______��
��2����Ӧװ�õ�ˮԡ�¶ȿ�����85�����ң�������130�棬����Ϊ______��Ե�ʣ�
������Ʊ���Ʒ
��3��Ϊ���ᴿ����ϩ����ȥ��Ʒ�еĻ��������������ˮ�����ʣ��������²�����
�ټ��뱥��ʳ��ˮ���������á��ֲ㡢��Һ������ʳ�ο���______���ã�
�ڷ�Һ������______������ţ��Լ�ϴ�ӣ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
�������Ʒ�м�����ˮ�Ȼ��ƺ���������ˮ�Ȼ���Ŀ���ǣ�______��
��4���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ������������ǣ�______������ţ���
A�������Ը��������Һ B���ý����� C���ⶨ�е㣮

��֪��
��Ũ���������ˮ�ԣ��ڼ���������Ҳ����̼��ͭ�ȵ��ʷ�Ӧ��
�����ɻ���ϩ�ķ�Ӧ��

����Ӧ���ʲ�������
ij��ѧС������ͼ��ʾװ���Ի�����Ϊԭ���Ʊ�����ϩ���䲽�����£�
������Ʊ���Ʒ
��1����12.5mL������������ͼ��Ӧװ���е�Բ����ƿA�ڣ��ټ���2mL��������Ϊ85%��Ũ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ������ƿD�ڵõ�����ϩ��Ʒ����������B��������______���˷�Ӧ�в���Ũ�����ԭ����______��
��2����Ӧװ�õ�ˮԡ�¶ȿ�����85�����ң�������130�棬����Ϊ______��Ե�ʣ�
������Ʊ���Ʒ
��3��Ϊ���ᴿ����ϩ����ȥ��Ʒ�еĻ��������������ˮ�����ʣ��������²�����
�ټ��뱥��ʳ��ˮ���������á��ֲ㡢��Һ������ʳ�ο���______���ã�
�ڷ�Һ������______������ţ��Լ�ϴ�ӣ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
�������Ʒ�м�����ˮ�Ȼ��ƺ���������ˮ�Ȼ���Ŀ���ǣ�______��
��4���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ������������ǣ�______������ţ���
A�������Ը��������Һ B���ý����� C���ⶨ�е㣮

��Ũ���������ˮ�ԣ��ڼ���������Ҳ����̼��ͭ�ȵ��ʷ�Ӧ��
�����ɻ���ϩ�ķ�Ӧ��

����Ӧ���ʲ�������
| �л��� | �ܶ�/ g?cm-3 | �۵�/ �� | �е�/ �� | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
������Ʊ���Ʒ
��1����12.5mL������������ͼ��Ӧװ���е�Բ����ƿA�ڣ��ټ���2mL��������Ϊ85%��Ũ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ������ƿD�ڵõ�����ϩ��Ʒ����������B��������______���˷�Ӧ�в���Ũ�����ԭ����______��
��2����Ӧװ�õ�ˮԡ�¶ȿ�����85�����ң�������130�棬����Ϊ______��Ե�ʣ�
������Ʊ���Ʒ
��3��Ϊ���ᴿ����ϩ����ȥ��Ʒ�еĻ��������������ˮ�����ʣ��������²�����
�ټ��뱥��ʳ��ˮ���������á��ֲ㡢��Һ������ʳ�ο���______���ã�
�ڷ�Һ������______������ţ��Լ�ϴ�ӣ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
�������Ʒ�м�����ˮ�Ȼ��ƺ���������ˮ�Ȼ���Ŀ���ǣ�______��
��4���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ������������ǣ�______������ţ���
A�������Ը��������Һ B���ý����� C���ⶨ�е㣮