��Ŀ����
��11�֣�T��X��Y��Z��Q��R��WΪ���ڱ�ǰ������Ԫ�أ�ԭ���������ε���������ijЩԪ�ص������Ϣ���±���
|
Ԫ�� |
�����Ϣ |
|
T |
Tԭ�����������������������ֱ�����ԭ��������� |
|
X |
X�Ļ�̬ԭ���е���ռ������������ͬ��ԭ�ӹ������ÿ�ֹ���еĵ�������ͬ |
|
Z |
Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊ |
|
Q |
�ڸ�Ԫ�����������У�Q�Ļ�̬ԭ�ӵĵ�һ��������С |
|
R |
3p�ܼ�����1������ |
|
W |
W��һ�ֺ��ص�������Ϊ65��������Ϊ36 |
��1��X��Y��Q����Ԫ�صĵ縺���ɴ�С��˳���� ����Ԫ�ط��ű�ʾ����
��2��X��Yԭ�ӽ���γɵ�X3Y4���壬����ṹ����ʯ���ƣ���X3Y4������۵�Ƚ��ʯҪ ����ߡ������͡�����
��3��W2+�ĺ�������Ų�ʽΪ ��Ԫ��W������������е������Լ�������Ӧ�����ɳ����W��HCl��O2��WCl��HO2��HO2 �������ᣩ������һ���������Ҳ��һ�����ɻ������м��ߵĻ��ԡ�����˵�����ʾ�������
A����������O2 B��HO2�ڼ��в����ȶ�����
C������������HO2 D��1 molW�μӷ�Ӧ��1 mol���ӷ���ת��
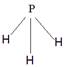
��4��X��Y��Z�ֱ�����Ԫ�ؿ��Թ���A��B��C��D�ȶ������ӡ�����A��B��C��Ϊ10��������DΪ18��������AΪ5ԭ�Ӻ˵�+1�������ӣ���A+������ԭ���ӻ���ʽΪ_______. BΪ4ԭ�Ӻ˵�+1�������ӣ���B+����ʽΪ___________��CΪ4��ԭ�Ӻ˹��ɵķ��ӣ�����C��Ϊ�ȵ�����ķ��ӿ�����_______��д�ṹʽ����D��������Ԫ�ص�ԭ�Ӹ���֮��Ϊ1:1����DΪ ������ԡ��Ǽ��ԡ������ӡ�ij˫ԭ�ӵ��ʷ���EҲΪ18��������E��ˮ�ķ�Ӧ�Ļ�ѧ����ʽΪ______________________��
��5����֪25�桢101 kPa�����£�
4R��s��+3Z2��g�� 2R2Z3��s�� ��H=-2835��9 kJ��mol
4R��s��+2Z3��g�� 2R2Z3��s�� ��H=-3119��1 kJ��mol
��16g Z2��g����ȫת��ΪZ3��g���ġ�H= ��
1��N��C��Na ��2���� ��3��[Ar]3d9��1s22s22p63s23p63d9��C
��4��sp3 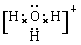
 ,
���� 2F2+2H2O=4HF+O2
,
���� 2F2+2H2O=4HF+O2
��5��+47��2 kJ/mol��2�֣�
������������Ԫ�صĽṹ�������ʿ�֪��T��X��Y��Z��Q��R��W�ֱ���H��C��N��O��Na��Al��Cu��
��1���ǽ�����Խǿ���縺��Խ������X��Y��Q����Ԫ�صĵ縺���ɴ�С��˳����N��C��Na��
��2��������ṹ����ʯ���ƣ�ʯī��ԭ�Ӿ��塣����̼ԭ�Ӱ뾶���ڵ�ԭ�Ӱ뾶��ԭ�Ӱ뾶ԽС�����ۼ�Խǿ�����Ըþ�����۵���ڽ��ʯ�ġ�
��3�����ݹ���ԭ����֪��ͭ���ӵĵ����Ų�ʽΪ[Ar]3d9��1s22s22p63s23p63d9�����ݷ���ʽ��֪��������������������HO2�ǻ�ԭ���ѡ��C����ȷ���������ȷ�ģ���ѡC��
��4��AΪ5ԭ�Ӻ˵�+1�������ӣ���A��NH4������ԭ����sp3�ӻ���BΪ4ԭ�Ӻ˵�+1�������ӣ���B��H3O���������ʽΪ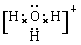 ��CΪ4��ԭ�Ӻ˹��ɵķ��ӣ���C�ǰ����������백����Ϊ�ȵ�����ķ��ӿ�����PH3����ṹʽΪ
��CΪ4��ԭ�Ӻ˹��ɵķ��ӣ���C�ǰ����������백����Ϊ�ȵ�����ķ��ӿ�����PH3����ṹʽΪ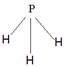 ��D��˫��ˮ�����ڼ��Է��ӡ�EӦ�����ǵ��ʷ�����ˮ��Ӧ�ķ���ʽΪ2F2+2H2O=4HF+O2��
��D��˫��ˮ�����ڼ��Է��ӡ�EӦ�����ǵ��ʷ�����ˮ��Ӧ�ķ���ʽΪ2F2+2H2O=4HF+O2��
��5�������˹���ɵ�Ӧ�á��٣��ڼ��õ�3Z2��g��
2Z3��g��
��H����283.2 kJ��mol������16g Z2��g����ȫת��ΪZ3��g���ġ�H����283.2 kJ��mol��6��+47��2 kJ/mol��

 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
