��Ŀ����
���ݻ�Ϊ2 L���ܱ������г���2 mol A�����1 mol B���壬��һ�������·������·�Ӧ��2A(g)��B(g) 3C(g)����2 s��ﵽƽ�⣬���C�����Ũ��Ϊ0.6 mol��L��1������˵������ȷ����
��������A��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ0.2 mol��L��1��s��1
��������B��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ0.2 mol��L��1��s��1
��ƽ��ʱ����A��B��ת�������
��ƽ��ʱ����B��Ũ��Ϊ0.2 mol��L��1
�������������䣬���������ټ���1 molC���壬�ﵽ��ƽ��ʱ��C�������������
A���٢ڢۡ�������B���٢ۢݡ�������C���ڢܢݡ�������D���٢ܢ�
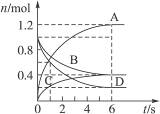
��������������淴Ӧ���йؼ��㡣�������͵�����������������ʽ����
2A(g)��B(g) 3C(g)
��ʼ����mol�� 2 1 0
ת������mol�� 0.8 0.4 1.2
ƽ������mol�� 1.2 0.6 1.2
����������A��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ
������B��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ
ƽ��ʱ����A��B��ת���ʷֱ�Ϊ![]() ��
��
ƽ��ʱ����B��Ũ��Ϊ![]()
����1mol��C���൱�ڼ���2/3molA��1/3molB����Ϊ��Ӧǰ��������䣬��ʱA��B�ı�ֵ��Ȼ����2�U1������ƽ���ǵ�Ч�ġ�
B

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д������ҹ�����ӵ�����ʽϿ��������ƣ�����β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�
|
 2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ�����ݻ�Ϊ2 L���ܱ������г���10 mol N2��5 mol O2���ﵽƽ���NO�����ʵ���Ϊ2 mol����T ��ʱ�÷�Ӧ��ƽ�ⳣ��K= �� ��������������С�������λ���֣�
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ�����ݻ�Ϊ2 L���ܱ������г���10 mol N2��5 mol O2���ﵽƽ���NO�����ʵ���Ϊ2 mol����T ��ʱ�÷�Ӧ��ƽ�ⳣ��K= �� ��������������С�������λ���֣� ��һ������NO�����ֽ�Ĺ����У�NO��ת������ʱ��仯����������ͼ��ʾ��
����֪��T1<T2��
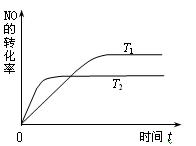
�ٷ�Ӧ 2NO(g)
 N2(g)+O2(g)Ϊ(����ȡ����ȡ�) �� ��Ӧ��
N2(g)+O2(g)Ϊ(����ȡ����ȡ�) �� ��Ӧ����һ���¶��£��ܹ�˵����Ӧ 2NO(g)
 N2(g)+O2(g) �Ѵﵽƽ����ǣ�����ţ��� ��
N2(g)+O2(g) �Ѵﵽƽ����ǣ�����ţ��� ��a�������ڵ�ѹǿ�������仯 b��NO�ֽ�����ʺ�NO���ɵ��������
c��NO��N2��O2��Ũ�ȱ��ֲ��� d����λʱ���ڷֽ�4 mol NO��ͬʱ����2 mol N2
�Ǣٵ�����������ϡ��ȼ��ʱ��β���е���Ҫ��Ⱦ��ΪNOx������CxHy����������ԭNOx���������������Ⱦ��
��֪��CH4(g)��4NO2(g) �� 4NO(g)��CO2(g)��2H2O(g) ��H1����574 kJ��mol-1
CH4(g)��4NO(g) �� 2N2(g)��CO2(g)+2H2O(g) ��H2
CH4(g)��2NO2(g) �� N2(g)��CO2(g)��2H2O(g) ��H3����867 kJ��mol-1
��H2�� �� ��
��ʹ�ô������Խ�����β������Ҫ�к��ɷ�һ����̼(CO)�͵�������(NOx)ת��Ϊ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ �� ��
 2NO (g)���÷�Ӧ�ǵ�������β���к���NO��ԭ��֮һ��
2NO (g)���÷�Ӧ�ǵ�������β���к���NO��ԭ��֮һ��