��Ŀ����
��1����KCl��CaCl2�Ļ�����У�K+��Ca2+�����ʵ���֮��Ϊ2��1����KCl��CaCl2�����ʵ���֮��Ϊ
��2����������Ϊ4��7��SO2��N2�Ļ�������У�SO2���������Ϊ
��3������β��������NO2������һ�������º�CO��Ӧ���������ʣ��䷴Ӧ����ʽ�ɱ�ʾΪ��2NO2+4CO N2+4CO2���÷�Ӧ����������
2��1
2��1
������֮��Ϊ149��111
149��111
����1mol�����ӵĸû���������Ϊ64.5g
64.5g
����2����������Ϊ4��7��SO2��N2�Ļ�������У�SO2���������Ϊ
20%
20%
����3������β��������NO2������һ�������º�CO��Ӧ���������ʣ��䷴Ӧ����ʽ�ɱ�ʾΪ��2NO2+4CO N2+4CO2���÷�Ӧ����������
NO2
NO2
������1molN2���ɣ���Ӧ��ת�Ƶ���Ϊ8
8
mol����������1���ɻ�ѧʽ��֪n��KCl��=n��K+����n��CaCl2��=n��Ca2+�����ݴ��ж�KCl��CaCl2�����ʵ���֮�ȣ�����m=nM������ߵ����������������������֮�ȣ����Ȼ��Ƶ����ʵ���Ϊxmol�����Ȼ���Ϊ2xmol�������������з��̼���x��ֵ������������Ե����ʵ������ٸ���m=nM��������������������������
��2����SO2��N2�������ֱ�Ϊ4g��7g������n=
����������ʵ���������������������ڼ��㣻
��3������Ԫ�ػ��ϼ۽��͵�����������������Ӧ��ֻ��NԪ�صĻ��ϼ۽��ͣ���+4�۽���Ϊ0�ۣ���NO2����������ת�Ƶ������ʵ����ǵ�4����
��2����SO2��N2�������ֱ�Ϊ4g��7g������n=
| m |
| M |
��3������Ԫ�ػ��ϼ۽��͵�����������������Ӧ��ֻ��NԪ�صĻ��ϼ۽��ͣ���+4�۽���Ϊ0�ۣ���NO2����������ת�Ƶ������ʵ����ǵ�4����
����⣺��1���ɻ�ѧʽ��֪n��KCl��=n��K+����n��CaCl2��=n��Ca2+������n��KCl����n��CaCl2��=n��K+����n��Ca2+��=2��1����m��KCl����m��CaCl2��=2mol��74.5g/mol��1mol��111g/mol=149��111�����Ȼ��Ƶ����ʵ���Ϊxmol�����Ȼ���Ϊ2xmol�����������ӿ�֪��2x+2x=1�����x=0.25����m��KCl��=0.25mol��2��74.5mol/L=37.25g��m��CaCl2��=0.25mol��111g/mol/L=27.75g���ʺ�1mol�����ӵĸû���������Ϊ37.25g+27.75g=64.5g��
�ʴ�Ϊ��2��1��149��111��64.5g��
��2����SO2��N2�������ֱ�Ϊ4g��7g����SO2�����ʵ���Ϊ
=
mol��N2�����ʵ���Ϊ
=
mol����
=20%��
�ʴ�Ϊ��20%��
��3����Ӧ��ֻ��NԪ�صĻ��ϼ۽��ͣ���+4�۽���Ϊ0�ۣ���NO2����������ת�Ƶ������ʵ�����NO2��4�����ɷ���ʽ2NO2+4CO=N2+4CO2��֪������1molN2����Ҫ2molNO2����ת�Ƶ��ӵ����ʵ���Ϊ2mol��4=8mol���ʴ�Ϊ��NO2��8��
�ʴ�Ϊ��2��1��149��111��64.5g��
��2����SO2��N2�������ֱ�Ϊ4g��7g����SO2�����ʵ���Ϊ
| 4g |
| 64g/mol |
| 1 |
| 16 |
| 7g |
| 28g/mol |
| 1 |
| 4 |
| ||||
|
�ʴ�Ϊ��20%��
��3����Ӧ��ֻ��NԪ�صĻ��ϼ۽��ͣ���+4�۽���Ϊ0�ۣ���NO2����������ת�Ƶ������ʵ�����NO2��4�����ɷ���ʽ2NO2+4CO=N2+4CO2��֪������1molN2����Ҫ2molNO2����ת�Ƶ��ӵ����ʵ���Ϊ2mol��4=8mol���ʴ�Ϊ��NO2��8��
���������⿼�����ʵ������йؼ��㡢���û�ѧ�������йؼ���ȣ��ѶȲ���ע�ʽ��������������ã�

��ϰ��ϵ�д�
�����Ŀ
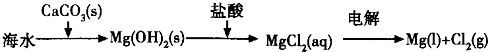
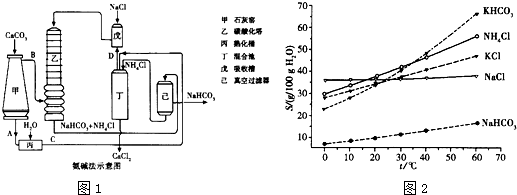
 ��2012?�Ͼ���ģ�������[Ca��IO3��2]��Ŀǰ�㷺ʹ�õļ��ܲ������ܲ��Ƶ�����ʳƷ���������Ӽ���
��2012?�Ͼ���ģ�������[Ca��IO3��2]��Ŀǰ�㷺ʹ�õļ��ܲ������ܲ��Ƶ�����ʳƷ���������Ӽ���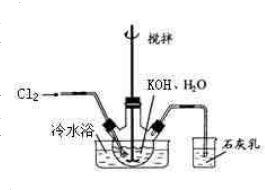 ��ˮԡ��ȴ��ͨ��Cl2���õ綯���������Ͻ��裬ֱ����Һ��Ϊ����ɫ����������ɫ��������Ϊֹ���ڽ����ʹ�����ص����ձ���������ˮԡ�У��ڲ��Ͻ����£��ּ��μ���130gKOH���壮�ò���ɰо©�����ˣ���ȥ��������Ҫ�ɷ�ΪKCl������KOH��KClO�����ô������ǿ���Ա�����Һ��
��ˮԡ��ȴ��ͨ��Cl2���õ綯���������Ͻ��裬ֱ����Һ��Ϊ����ɫ����������ɫ��������Ϊֹ���ڽ����ʹ�����ص����ձ���������ˮԡ�У��ڲ��Ͻ����£��ּ��μ���130gKOH���壮�ò���ɰо©�����ˣ���ȥ��������Ҫ�ɷ�ΪKCl������KOH��KClO�����ô������ǿ���Ա�����Һ�� 5KCl+KClO3+3H2O��
5KCl+KClO3+3H2O��