��Ŀ����
��11�֣��о��Ϳ���CO2��CO�Ĵ��������ǻ�����������Դ���õ�˫Ӯ�Ŀ��⡣
��1��CO�����ںϳɼ״�����ѹǿΪ0.1Mpa�����£������ΪbL���ܱ������г���amolCO��2amolH2���ڴ��������ºϳɼ״���
CO��g��+2H2��g��CH3OH��g��ƽ��ʱCO��ת�������¶ȣ�ѹǿ�Ĺ�ϵ����ͼ��
��i���÷�Ӧ����_____________��Ӧ��������ȡ����ȡ�����
��ii��100��ʱ���÷�Ӧ��ƽ�ⳣ����K=_____________������a��b�Ĵ���ʽ��ʾ������һ�����淴Ӧ��ƽ�ⳣ��Kֵ�ܴԴ˷�Ӧ��˵����ȷ���ǣ�_________________����ţ�
�÷�Ӧʹ�ô������岻��
�÷�Ӧ�������ںܶ�ʱ������ɣ�
�÷�Ӧ�ﵽƽ��ʱ������һ�ַ�Ӧ��ٷֺ�����С��
�÷�Ӧһ���Ƿ��ȷ�Ӧ��
��iii�����¶Ⱥ��ݻ����������£�����ƽ����ϵ�г���amolCO��2amolH2���ﵽƽ��ʱCOת����________������������䡱��С������ͬ��ƽ�ⳣ����________��
��iv����ij�¶��£���һ�ݻ�������ܱ������г���2.5molCO��7.5molH2����Ӧ����CH3OH��g�����ﵽƽ��ʱ��COת����Ϊ90%����ʱ������ѹǿΪ��ʼʱ��ѹǿ__________����
��2��ij�¶������£�����CO2��g����H2��g���������1��4��ϣ����ʵ�ѹǿ�ʹ��������¿��Ƶü��飬��֪��
CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3KJ/mol
H2��g��+1/2O2��g��= H2O��l�� ��H=-285.8KJ/mol
��CO2��g����H2��g����Ӧ����Һ̬ˮ���Ȼ�ѧ����ʽΪ��__________________________________________________________________________��
��1����i������ ��ii��b2/a2 C ��iii������ ���� ��iv��0.55
��2��CO2��g��+4 H2��g��= CH4��g��+ 2H2O��l����H=-252.9 KJ/mol
����:

 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д���11�֣��о��Ϳ���CO2��CO�Ĵ��������ǻ�����������Դ���õ�˫Ӯ�Ŀ��⡣
��1��CO�����ںϳɼ״�����ѹǿΪ0.1Mpa�����£������ΪbL���ܱ������г���amolCO��2amolH2���ڴ��������ºϳɼ״���
CO��g��+2H2��g�� CH3OH��g��ƽ��ʱCO��ת�������¶ȣ�ѹǿ�Ĺ�ϵ����ͼ��
CH3OH��g��ƽ��ʱCO��ת�������¶ȣ�ѹǿ�Ĺ�ϵ����ͼ��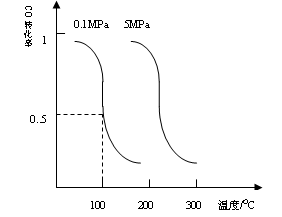
��i���÷�Ӧ����_____________��Ӧ��������ȡ����ȡ�����
��ii��100��ʱ���÷�Ӧ��ƽ�ⳣ����K=_____________������a��b�Ĵ���ʽ��ʾ����
��һ�����淴Ӧ��ƽ�ⳣ��Kֵ�ܴԴ˷�Ӧ��˵����ȷ���ǣ�_________________����ţ�
| A���÷�Ӧʹ�ô������岻�� |
| B���÷�Ӧ�������ںܶ�ʱ������ɣ� |
| C���÷�Ӧ�ﵽƽ��ʱ������һ�ַ�Ӧ��ٷֺ�����С�� |
| D���÷�Ӧһ���Ƿ��ȷ�Ӧ�� |
��iv����ij�¶��£���һ�ݻ�������ܱ������г���2.5molCO��7.5molH2����Ӧ����CH3OH��g�����ﵽƽ��ʱ��COת����Ϊ90%����ʱ������ѹǿΪ��ʼʱ��ѹǿ__________����
��2��ij�¶������£�����CO2��g����H2��g���������1��4��ϣ����ʵ�ѹǿ�ʹ��������¿��Ƶü��飬��֪��
CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3KJ/mol
H2��g��+1/2O2��g��= H2O��l�� ��H=-285.8KJ/mol
��CO2��g����H2��g����Ӧ����Һ̬ˮ���Ȼ�ѧ����ʽΪ��_________________________��
 ��2012?��������ģ���о��Ϳ���CO2��CO�Ĵ��������ǻ�����������Դ���õ�˫Ӯ���⣮CO�����ںϳɼ״�����ѹǿΪ0.1MPa�����£������Ϊb L���ܱ������г���a mol CO��2a mol H2���ڴ��������ºϳɼ״���CO��g��+2H2��g��?CH3OH��g����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��
��2012?��������ģ���о��Ϳ���CO2��CO�Ĵ��������ǻ�����������Դ���õ�˫Ӯ���⣮CO�����ںϳɼ״�����ѹǿΪ0.1MPa�����£������Ϊb L���ܱ������г���a mol CO��2a mol H2���ڴ��������ºϳɼ״���CO��g��+2H2��g��?CH3OH��g����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ�� (1)100��ʱ���÷�Ӧ��ƽ�ⳣ��:
(1)100��ʱ���÷�Ӧ��ƽ�ⳣ��: