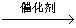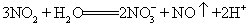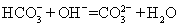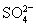��Ŀ����
һ��ⶨ��Ʒ�гɷֺ�����ʵ��Ӧ�ظ�2��3�Ρ�Ϊ�˲ⶨij�������ƹ����л��е�̼���Ƶ������������ס��ҡ�����λͬѧ�ֱ����������ʵ�鷽����

��ͬѧ�ķ�����ͼ��ʾ��
��1����μ���Aװ�õ������ԣ�_____________________________________________��
��2����ͬѧ�ظ�����������ʵ�飬�õ�̼���Ƶ��������������ݴ��ڽϴ��ƫ�����Ϊ��������������ƫ�͵�ԭ����_______������ţ���
A��װ����ԭ�п����еĶ�����̼����Ҳ����ʯ������
B��װ��������е�ˮ�����Ͷ�����̼����ʯ������
C����Ӧ��ɺ�װ���еĶ�����̼û��ȫ������ʯ������
D������ϡ����������㡢��Ӧ�����
��3��Ϊ���ü�ʵ����������ȷ��������ʵ�鲽�趼��ȷ�������£�����Ϊͼ�е�ʵ��װ��Ӧ����θĽ���______________��
����ͬѧ�ķ����ǣ���ͼ�����ṩ��װ����ѡ��ʵ��װ�ã������ͬѧʵ��װ���е�B��C��ͨ���ⶨ�ų��Ķ�����̼������������Ƕ�����̼����ˮ�������㡣

ѡ�����װ�õ�����˳��Ϊ_______��
��ͬѧ�ķ����ǣ���ȡ��Ʒm g�����ܽ⣬��������Ȼ�����Һ�����ˡ�ϴ�ӡ���ɡ��������ù���n g��
��1������100 mL 0��10 mol/L BaCl2��Һ��ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ���_______�����������ƣ���
��2���������̼���Ƶ���������Ϊ����m��n��ʾ��_______��
��3��Ca2+��Ba2+������ʹ ������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��
������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��
��1����ֹˮ�мн�Aװ�õ�����ĩ�˵���Ƥ�ܣ���Һ©���ϲ������ӣ�������ע����������ˮ����������ʼ������ˮ���£���һ�����ˮ���ܵ���Բ����ƿ��֤��װ������������
��2��C��D ��3����װ��ʯ�ҵĸ�����ұ���װһ��ʢ�м�ʯ�ҵĸ���ܣ���ֹ������ˮ�ֺͶ�����̼��Cװ���еļ�ʯ������ �ݢߢ�
��1��100 mL����ƿ
��2��106n/197m
��3���� ������Ca2+����OH-��������ˮ���������Ƴ�����Ӱ����
����������1������Aװ�������Եķ�������ֹˮ�мн�Aװ�õ�����ĩ�˵���Ƥ�ܣ���Һ©���ϲ������ӣ�������ע����������ˮ����������ʼ������ˮ���£���һ�����ˮ���ܵ���Բ����ƿ��֤��װ�����������á���2����װ����ԭ�п����е�CO2���屻��ʯ������ʱ�����CO2�����������²������ƫ�ߣ���װ��������е�ˮ������CO2����ʯ������ʱ�����CO2�����������²������ƫ�ߣ���װ���еĶ�����̼û��ȫ������ʯ������ʱ�����CO2���������٣����²������ƫ�ͣ�������ϡ����������㣬��Ӧ�����ʱ��������CO2���٣����CO2���������٣����²������ƫ�͡���3��Ϊ���ü�ʵ����������ȷ��Ӧ��װ��ʯ�ҵĸ�����ұ���װһ��ʢ�м�ʯ�ҵĸ���ܣ���ֹ������ˮ�ֺͶ�����̼��Cװ���еļ�ʯ�����ա�
����������װ��֪���ⶨCO2��������Ӧ�á���ˮ��������������ѡ��װ�âݡ��ޡ��ߣ������d�ڽ�����c�ڳ�ˮ��ˮ��װ�â߽���װ�âޡ���1������һ�����ʵ���Ũ�ȵ���Һ���õ��IJ��������У��ձ�����������100 mL����ƿ����ͷ�ιܡ���Ͳ����2��n��Na2CO3��=n��BaCO3��= =
=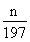 mol����Na2CO3������������
mol����Na2CO3������������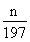 mol��106 g/mol��m g=106n/197m��
mol��106 g/mol��m g=106n/197m��
��3�����ڹ�����Ca2+����OH-��������ˮ���������Ƴ�����Ӱ�������������Բ���ʹ���Ȼ�����Һ�����Ȼ�����Һ��