��Ŀ����
����Ŀ����֪��A��B��C��D���ֶ�����Ԫ�أ�A��D��ԭ������֮�͵���B��C��ԭ������֮�ͣ���DԪ����ɵĵ�����ͨ��״���³ʻ���ɫ��B��C��D����Ԫ��λ��ͬһ���ڣ�A��B��C����Ԫ�ص�����������Ӧ��ˮ����ֱ�ΪX��Y��Z���Ҵ�������ͼת����ϵ�����ƶϻش��������⡣
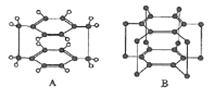
��1��DԪ��ԭ�ӵĽṹʾ��ͼΪ_____________��
��2��A��B��C����Ԫ�ص�ԭ�Ӱ뾶��С�����˳��Ϊ______________����Ԫ�ط��ű�ʾ����
��3��Y��CԪ�ص������������Է�����Ӧ���÷�Ӧ�����ӷ���ʽΪ_______________��
��4��A����̬�⻯��ĵ���ʽ___________________ ��
��5��ʵ�����У���ȡA����̬�⻯��Ļ�ѧ����ʽ _________________________________��
���𰸡� ![]() N<Al<Na Al2O3+2OH-=2AlO2- +H2O
N<Al<Na Al2O3+2OH-=2AlO2- +H2O 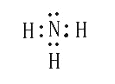
![]()
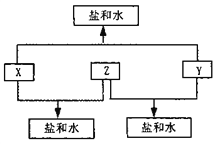
��������A��B��C��D���ֶ�����Ԫ�أ�A��D��ԭ������֮�͵���B��C��ԭ������֮�ͣ���DԪ����ɵĵ�����ͨ��״���³ʻ���ɫ������D��ClԪ�أ�B��C��D����Ԫ��λ��ͬһ���ڣ��ڷ�Ӧ��B��C����������ˮ���������κ�ˮ������B��CӦ���ǵ������ڵĽ���Ԫ�أ�Ҳ����˵B��CӦ������þ��֮��ģ�����ΪY+Z=�κ�ˮ��X+Z=��+ˮ������Z������������C�����������������Ժ�ǿ������ƫ�����κ�ˮ������BӦ�����ƣ�C������A��D��ԭ������֮�͵���B��C��ԭ������֮�ͣ��ó�A�ǵ���
(1)DԪ������Ԫ�أ���ԭ�ӽṹʾ��ͼΪ 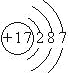 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(2)���Ӳ���Խ�࣬ԭ�ӵİ뾶Խ���Ӳ�����ͬ��ԭ�ӣ�ԭ�Ӱ뾶����ԭ���������������С������ԭ�Ӱ뾶��С�����˳��ΪN��Al��Na���ʴ�Ϊ��N��Al��Na��
(3)Y��ǿ�C��������������������������ǿ�Ӧ����ƫ�����Σ����ӷ���ʽΪ��Al2O3+2OH-=2AlO2- +H2O���ʴ�Ϊ��Al2O3+2OH-=2AlO2- +H2O��
(4)A����̬�⻯��Ϊ�����������ĵ���ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)ʵ�����У���ȡ�����Ļ�ѧ����ʽ Ϊ2NH4Cl + Ca(OH)2![]() CaCl2 + 2NH3��+2H2O���ʴ�Ϊ��2NH4Cl + Ca(OH)2
CaCl2 + 2NH3��+2H2O���ʴ�Ϊ��2NH4Cl + Ca(OH)2![]() CaCl2 + 2NH3��+2H2O��
CaCl2 + 2NH3��+2H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�