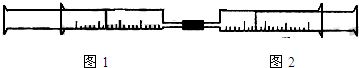
��Ŀ����
��ͼ��ʾ����ͼ1��ͼ2����װ�в�ͬ���ʵ���Ͳ�õ���������������ͼ2��Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ����ҺŨ����ͬ����
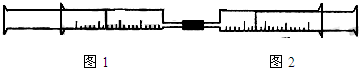
�Իش��������⣺
��1��ʵ��1�У��������ձ�Ϊ
��2��ʵ��2ͼ1��Ͳ�ڵ������ǣ�
��3��ʵ��3ͼ1��Ͳ�ڵ������ǣ�
��4��ʵ��4�У���֪��3Cl2+2NH3=N2+6HCl��ͼ1��Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ

�Իش��������⣺
| ʵ����� | ͼ1��Ͳ������ | ͼ2��Ͳ������ | ͼ1��Ͳ������ |
| 1 | 10mLFeSO4��Һ | 10mLNH3 | ���ɰ�ɫ���������ɫ |
| 2 | 10mL1mol/LAlCl3��Һ | 40mL1mol/LNaOH��Һ | ���а�ɫ������������ܽ� ���а�ɫ������������ܽ� |
| 3 | 10mL��ɫʯ����Һ | 25mLCl2 | �ȱ�����ɫ �ȱ�����ɫ |
| 4 | 15mLCl2 | 40mLNH3 | ����ɫ��Ϊ��ɫ ����ɫ��Ϊ��ɫ |
���
���
ɫ��д��������ɫ�Ļ�ѧ����ʽ4Fe��OH��2+O2+2H2O=4Fe��OH��3
4Fe��OH��2+O2+2H2O=4Fe��OH��3
����2��ʵ��2ͼ1��Ͳ�ڵ������ǣ�
���а�ɫ������������ܽ�
���а�ɫ������������ܽ�
��д���йط�Ӧ�����ӷ���ʽ��Al3++3OH-=Al��OH��3��
Al3++3OH-=Al��OH��3��
��Al��OH��3+OH-=AlO2-+2H2O
Al��OH��3+OH-=AlO2-+2H2O
����3��ʵ��3ͼ1��Ͳ�ڵ������ǣ�
�ȱ�����ɫ
�ȱ�����ɫ
����Ӧ��ͼ2��Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��NaOH
NaOH
��Һ�У���4��ʵ��4�У���֪��3Cl2+2NH3=N2+6HCl��ͼ1��Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ
����ɫ��Ϊ��ɫ
����ɫ��Ϊ��ɫ
����������1��+2�۵��������ױ�����������+3�۵������ӣ�NH3��FeSO4��Һ��ˮ��Ӧ���ɰ�ˮ����ˮ�����OH-��FeSO4��Һ��+2�۵������ӷ�Ӧ����Fe��OH��2��Fe��OH��2�������е�����������
��2��Al��OH��3�������ԣ���ʽ���룺Al��OH��3?Al3++3OH-��ʽ���룺Al��OH��3?H2O+H++AlO2-��������ǿ�ᣬ��������ǿ�
��3����ˮ�е�HClO����ǿ�����ԣ���Ư��ָʾ��������������NaOH���գ�
��4����������ɫΪ����ɫ��3Cl2+2NH3�TN2+6HCl������İ���������Ȼ��ⷴӦ��
��2��Al��OH��3�������ԣ���ʽ���룺Al��OH��3?Al3++3OH-��ʽ���룺Al��OH��3?H2O+H++AlO2-��������ǿ�ᣬ��������ǿ�
��3����ˮ�е�HClO����ǿ�����ԣ���Ư��ָʾ��������������NaOH���գ�
��4����������ɫΪ����ɫ��3Cl2+2NH3�TN2+6HCl������İ���������Ȼ��ⷴӦ��
����⣺��1��NH3+H2O?NH3?H2O?NH4++OH-��FeSO4+2NH3?H2O�TFe��OH��2��+��NH4��2SO4��Fe��OH��2Ϊ��ɫ�������ڿ������ױ������е�����������������Ӧ��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��Fe��OH��3Ϊ���ɫ��
�ʴ�Ϊ����֣�4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��2��10mL1mol/L��AlCl3��Һ��40mL1mol/L��NaOH��Һ��Ӧ��0.01molAlCl3��0.04molNaOH ��Ӧ������
Al3++3OH-=Al��OH��3�� Al��OH��3 +OH -=AlO2-+2H2O
0.01mol 0.03mol 0.01mol 0.01mol 0.01mol 0.01mol
����Ϊ�����а�ɫ������������ܽ⣮
�ʴ�Ϊ�����а�ɫ������������ܽ⡢Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O��
��3��Cl2+H2O?HCl+HClO��HCl��HClO���������ԣ���ʹ��ɫʯ����Һ��죬HClO����ǿ�����ԣ���Ư��ָʾ����ɫ��ȥ��Cl2+2NaOH�TNaCl+H2O+NaClO
����������NaOH���գ�
�ʴ�Ϊ���ȱ�����ɫ��NaOH��
��4�����ݷ���ʽ��֪��3Cl2+2NH3�TN2+6HCl
15mL 10mL 5mL 30mL
����30mLHCl��ʣ���NH3Ϊ30mL��
��NH3+HCl�TNH4Cl
30mL 30mL
��֪�����ɵ�HCl��ʣ���NH3ǡ����ȫ��Ӧ����NH4Cl�����ŷ�Ӧ�Ľ��У�Cl2�������٣�������ȫ��Ӧ��
�������ʣ�������Ϊ5mL��ɫ��N2��
�ʴ�Ϊ������ɫ��Ϊ��ɫ��
�ʴ�Ϊ����֣�4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��2��10mL1mol/L��AlCl3��Һ��40mL1mol/L��NaOH��Һ��Ӧ��0.01molAlCl3��0.04molNaOH ��Ӧ������
Al3++3OH-=Al��OH��3�� Al��OH��3 +OH -=AlO2-+2H2O
0.01mol 0.03mol 0.01mol 0.01mol 0.01mol 0.01mol
����Ϊ�����а�ɫ������������ܽ⣮
�ʴ�Ϊ�����а�ɫ������������ܽ⡢Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O��
��3��Cl2+H2O?HCl+HClO��HCl��HClO���������ԣ���ʹ��ɫʯ����Һ��죬HClO����ǿ�����ԣ���Ư��ָʾ����ɫ��ȥ��Cl2+2NaOH�TNaCl+H2O+NaClO
����������NaOH���գ�
�ʴ�Ϊ���ȱ�����ɫ��NaOH��
��4�����ݷ���ʽ��֪��3Cl2+2NH3�TN2+6HCl
15mL 10mL 5mL 30mL
����30mLHCl��ʣ���NH3Ϊ30mL��
��NH3+HCl�TNH4Cl
30mL 30mL
��֪�����ɵ�HCl��ʣ���NH3ǡ����ȫ��Ӧ����NH4Cl�����ŷ�Ӧ�Ľ��У�Cl2�������٣�������ȫ��Ӧ��
�������ʣ�������Ϊ5mL��ɫ��N2��
�ʴ�Ϊ������ɫ��Ϊ��ɫ��
�����������漰Ԫ�ػ����������ȡ��������ʣ��ۺ���ǿ����ѧϰ�ý�֪ʶʱ������صķ�Ӧ����ʽ��

��ϰ��ϵ�д�
 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
�����Ŀ
��ͼ��ʾ����ͼ1��ͼ2����װ�в�ͬ���ʵ���Ͳ�õ���������������ͼ2��Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ����ҺŨ����ͬ����

�Իش��������⣺
| ʵ����� | ͼ1��Ͳ������ | ͼ2��Ͳ������ | ͼ1��Ͳ������ |
| 1 | 10mLFeSO4��Һ | 10mLNH3 | ���ɰ�ɫ���������ɫ |
| 2 | 10mL1mol/LAlCl3��Һ | 40mL1mol/LNaOH��Һ | ______ |
| 3 | 10mL��ɫʯ����Һ | 25mLCl2 | ______ |
| 4 | 15mLCl2 | 40mLNH3 | ______ |
��2��ʵ��2ͼ1��Ͳ�ڵ������ǣ�______��д���йط�Ӧ�����ӷ���ʽ��______��______��
��3��ʵ��3ͼ1��Ͳ�ڵ������ǣ�______����Ӧ��ͼ2��Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��______��Һ�У�
��4��ʵ��4�У���֪��3Cl2+2NH3=N2+6HCl��ͼ1��Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ______��
��ͼ��ʾ����ͼ1��ͼ2����װ�в�ͬ���ʵ���Ͳ�õ���������������ͼ2��Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ����ҺŨ����ͬ����

�Իش��������⣺
��1��ʵ��1�У��������ձ�Ϊ______ɫ��д��������ɫ�Ļ�ѧ����ʽ______��
��2��ʵ��2ͼ1��Ͳ�ڵ������ǣ�______��д���йط�Ӧ�����ӷ���ʽ��______��______��
��3��ʵ��3ͼ1��Ͳ�ڵ������ǣ�______����Ӧ��ͼ2��Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��______��Һ�У�
��4��ʵ��4�У���֪��3Cl2+2NH3=N2+6HCl��ͼ1��Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ______��

�Իش��������⣺
| ʵ����� | ͼ1��Ͳ������ | ͼ2��Ͳ������ | ͼ1��Ͳ������ |
| 1 | 10mLFeSO4��Һ | 10mLNH3 | ���ɰ�ɫ���������ɫ |
| 2 | 10mL1mol/LAlCl3��Һ | 40mL1mol/LNaOH��Һ | ______ |
| 3 | 10mL��ɫʯ����Һ | 25mLCl2 | ______ |
| 4 | 15mLCl2 | 40mLNH3 | ______ |
��2��ʵ��2ͼ1��Ͳ�ڵ������ǣ�______��д���йط�Ӧ�����ӷ���ʽ��______��______��
��3��ʵ��3ͼ1��Ͳ�ڵ������ǣ�______����Ӧ��ͼ2��Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��______��Һ�У�
��4��ʵ��4�У���֪��3Cl2+2NH3=N2+6HCl��ͼ1��Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ______��
 2SO3(g)��ƽ������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ��ͼ
2SO3(g)��ƽ������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ��ͼ ��ʾ������ͼ�ش��������⣺
��ʾ������ͼ�ش��������⣺