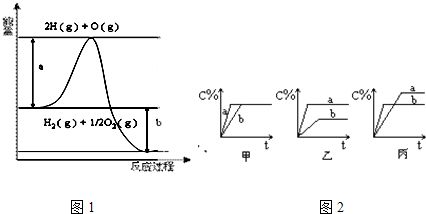
��Ŀ����
��8�֣���1����֪H2��g��ȼ����Ϊ-285��8kJ��mol-1��̫���ֽܷ�10molˮ���ĵ�������_____________kJ��
��2����֪0.4 molҺ̬��(N2 H4)������H2O2��Ӧ�����ɵ�����ˮ�������ų�256.65 kJ��������д���ø÷�Ӧ���Ȼ�ѧ����ʽ__________________________________________________��
����֪ H2O��l��=== H2O��g����H����44kJ��mol��1��16gҺ̬��������H2O2��Ӧ�����ɵ�����Һ̬ˮ�ų���������_____________ kJ��
������ӦӦ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ����ͻ�����ŵ���____________________________________________________________��
��8�֣�ÿ��2�֣���1��2858
��2��N2H4(l)+2H2O2(l)=N2(g)+4H2O(g) ��H=��641.625kJ/mol
408.8 ���ɵ�N2�� H2O���Ի�������Ⱦ��
����

��ϰ��ϵ�д�
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
�����Ŀ
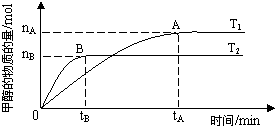 ��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��