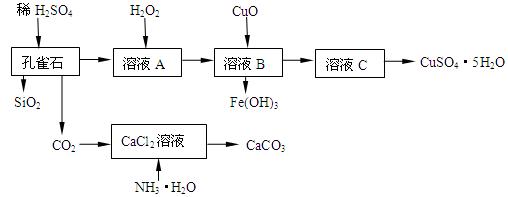
��Ŀ����
��98%��ŨH2SO4����=1.84g/cm3������500ml0.5mol/L��ϡH2SO4���밴Ҫ����գ�
������ŨH2SO4�����Ϊ
�����ʵ������10mL��20mL��50mL��Ͳ��Ӧѡ�� mL��Ͳ��ʵ���л���Ҫ�õ���������
����ʵ���г������������������ҺŨ����ʲôӰ�죿����ƫ�ߡ�ƫ�͡���Ӱ�죩
��Ũ�����ܽ��δ�������¼����ж���
�ڶ���ʱ���ӿ̶���
��������ʱҺ����ڿ̶���Ӧ��ȡ�Ĵ�ʩ��
������ŨH2SO4�����Ϊ
�����ʵ������10mL��20mL��50mL��Ͳ��Ӧѡ�� mL��Ͳ��ʵ���л���Ҫ�õ���������
����ʵ���г������������������ҺŨ����ʲôӰ�죿����ƫ�ߡ�ƫ�͡���Ӱ�죩
��Ũ�����ܽ��δ�������¼����ж���
�ڶ���ʱ���ӿ̶���
��������ʱҺ����ڿ̶���Ӧ��ȡ�Ĵ�ʩ��
�� 13.6ml �� 20 mL�� 500ml����ƿ �� ��ͷ�ι�
�� �� ƫ��
�� ƫ��
�� ��������
�� �� ƫ��
�� ƫ��
�� ��������
��

��ϰ��ϵ�д�
�����Ŀ


 ȷ���� ��
ȷ���� �� ��ˮ
��ˮ