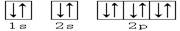��Ŀ����
��18�֣���֪X��Y��Z��W����Ԫ�طֲ���Ԫ�����ڱ��е�������ͬ������Ԫ�����ԭ��������������X��Wͬ���壬Y��ZΪͬ���ڵ�����Ԫ�ء�Wԭ�ӵ�����������Y��Zԭ������������֮�͡�Y���⻯���������3�����ۼ���Zԭ�������������Ǵ�����������3�������ƶϣ�
��1��д��X��Y��Ԫ�ط��ţ�X ��Y ��
��2��W��Ԫ�����ڱ��е�λ���� ��Z��ԭ�ӽṹʾ��ͼ
��3����X��Y��Z���γɵ����ӻ�����Ļ�ѧʽ�� ������W������������ˮ�������Һ��Ӧʱ�����ӷ���ʽ�� ��
��4���õ���ʽ��ʾY���⻯����γɹ��� ��
��1��д��X��Y��Ԫ�ط��ţ�X ��Y ��
��2��W��Ԫ�����ڱ��е�λ���� ��Z��ԭ�ӽṹʾ��ͼ
��3����X��Y��Z���γɵ����ӻ�����Ļ�ѧʽ�� ������W������������ˮ�������Һ��Ӧʱ�����ӷ���ʽ�� ��
��4���õ���ʽ��ʾY���⻯����γɹ��� ��
��1��H (2��) N(2��)
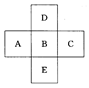
��2���������ڵڢ�A��(3��)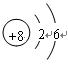 (2��)
(2��)
��3��NH4NO3 (3��) NH4+ + OH- ="== " NH3��H2O (3��)
��4��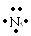 + 3
+ 3 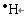

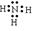 (3��)
(3��)
��2���������ڵڢ�A��(3��)
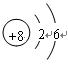 (2��)
(2��) ��3��NH4NO3 (3��) NH4+ + OH- ="== " NH3��H2O (3��)
��4��
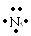 + 3
+ 3 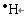

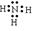 (3��)
(3��)��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
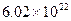 ����ԭ��/cm3������Ӧ���£�LaNi5H6
����ԭ��/cm3������Ӧ���£�LaNi5H6 LaLi5+3H2�����й���LaNi5H6���е���أ�����Ϊ�������⻯�
LaLi5+3H2�����й���LaNi5H6���е���أ�����Ϊ�������⻯�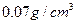 ������ͬ���LaNi5��������������ƣ���Һ̬��ĺ�����֮��Ϊ ��
������ͬ���LaNi5��������������ƣ���Һ̬��ĺ�����֮��Ϊ ��