��Ŀ����
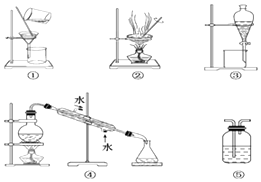
����Ŀ����ҵ���Ժ����ƼΪ�����������������ƣ�Na2S2O5�������Ʊ�����������ͼ��

��֪����ӦII����2NaHSO3=Na2S2O5+H2O�ȶಽ��Ӧ��
��1����ӦI�Ļ�ѧ����ʽΪ___________________________________________��
��2����������ʱ������Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
��3����֪Na2S2O5��ϡ���ᷴӦ�ų�SO2�������ӷ���ʽΪ________________��
��4����ӦIʱӦ��ͨ������Ϊ__________������ƷX�Ļ�ѧʽ��_________________��
��5��Ϊ�˼��ٲ�ƷNa2S2O5�����ʺ���������Ʒ�ӦII���������������ʵ���֮��ԼΪ______��
���𰸡� NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl 2CuS+3O2����2CuO+2SO2 S2O52-+2H+=2SO2��+H2O NH3 CuSO45H2O 2�U1
��������������Ҫ���������ҵ���Ժ����ƼΪ�����������������ƣ�Na2S2O5���Ĺ������̵����ۡ�
��1����ӦI�Ļ�ѧ����ʽΪNaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��������ΪNH3+CO2+H2O=NH4HCO3��NaCl+ NH4HCO3=NaHCO3��+NH4Cl��
��2����������ʱ���ɵĺ�ɫ����������ͭ��ͬʱSת��ΪSO2��������Ӧ�Ļ�ѧ����ʽΪ2CuS+3O2![]() 2CuO+2SO2��
2CuO+2SO2��
��3����֪Na2S2O5��ϡ���ᷴӦ�ų�SO2�������ӷ���ʽΪS2O52-+2H+=2SO2��+H2O��
��4��NH3���ܽ�ȴ���CO2�����Է�ӦIʱӦ��ͨ������ΪNH3���������ɫ��������ͭ��Ӧ��������ͭ������ƷX�Ļ�ѧʽ��CuSO45H2O��
��5��Ϊ�˼��ٲ�ƷNa2S2O5�����ʺ������ӻ�ѧʽNa2S2O5���Կ�������Ʒ�ӦII���������������ʵ���֮��ԼΪ2:1��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�