��Ŀ����
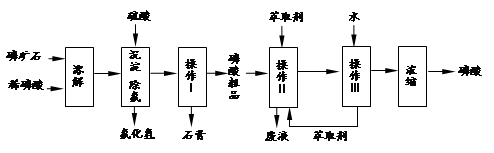
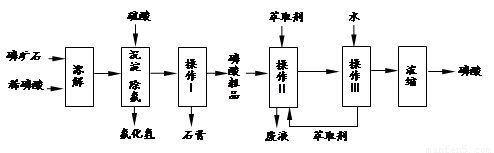
��ʯ����Ҫ�ɷ���Ca5F��PO4��3��������MgO��Fe2O3�����ʣ���ҵ������ʯΪԭ���Ʊ�H3PO4�ij����������£�
��֪��Ca5F��PO4��3+7H3PO4��5Ca ��H2PO4��2+HF
��1���������ַ�����ʵ�����ܽ���ʯ ����ܡ����ܡ����ò���������ԭ���� ��
��2��������������� �����������Ʒ�г���H+�⣬�����е��������� ��
��3����ʵ������ʵ�ֲ�����͢�����Ҫ�IJ��������� ���Ʋ����ȡ��һ�����е������� ��
a������ȡ����ˮ�������� b����ͬ�����£�����ȡ�����ܶȱ�ˮС
c�������ڸ���ȡ���е��ܽ�Ⱥ�С d��ijЩ����������ڸ���ȡ���е��ܽ�Ⱥ�С
��4�����ø����̳���ȡ�����⣬���� �ȸ���Ʒ��������˵������һ�ָ���Ʒ����; ��
��5����ֱ���������ܽ���ʯ�Ĺ�����ȣ��ù��յ��ŵ��� ��
��6���ⶨ�����Ʒ��Ũ�ȿɲ��õζ�����ȷ��ȡ10.00mL�����Ʒ���ܶ�Ϊ1.526g/cm3������ˮ���1L��Һ��ȡ�ܽ�����Һ20.00mL���Լ�����ָʾ������0.103mol/L ��NaOH��Һ�ζ����յ㣨����NaH2PO4��������NaOH��Һ21.35mL���������Ʒ����������Ϊ ��

��֪��Ca5F��PO4��3+7H3PO4��5Ca ��H2PO4��2+HF
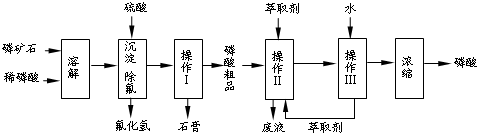
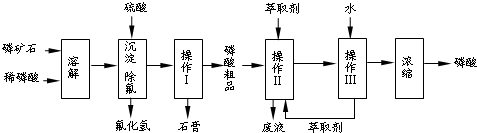
��1���������ַ�����ʵ�����ܽ���ʯ
��2���������������
��3����ʵ������ʵ�ֲ�����͢�����Ҫ�IJ���������
a������ȡ����ˮ�������� b����ͬ�����£�����ȡ�����ܶȱ�ˮС
c�������ڸ���ȡ���е��ܽ�Ⱥ�С d��ijЩ����������ڸ���ȡ���е��ܽ�Ⱥ�С
��4�����ø����̳���ȡ�����⣬����
��5����ֱ���������ܽ���ʯ�Ĺ�����ȣ��ù��յ��ŵ���
��6���ⶨ�����Ʒ��Ũ�ȿɲ��õζ�����ȷ��ȡ10.00mL�����Ʒ���ܶ�Ϊ1.526g/cm3������ˮ���1L��Һ��ȡ�ܽ�����Һ20.00mL���Լ�����ָʾ������0.103mol/L ��NaOH��Һ�ζ����յ㣨����NaH2PO4��������NaOH��Һ21.35mL���������Ʒ����������Ϊ
��������1��HF���и�ʴ�ԣ��ܹ���ʴ����������ʹ�ò������ܽ���ʯ��
��2��������ͨ�����˷����ʯ�ࣻ����ͼʾ�������Ʒ�к���H+��Ca2+��Mg2+��Fe3+���ӣ�
��3��������ȡ�����õ���������ɣ�������ȡ�����ж���ȡ��Ҫ�������
��4���Ʊ�H3PO4�ij������̿���֪��������ȡ�����⣬���з����⡢ʯ��ȸ���Ʒ�����ݷ������������ʯ�����;��ɣ�
��5��ֱ���������ܽ���ʯ�����ɵ�ʯ����������ʯ���棻
��6���������ɲ���NaH2PO4���������ƵĹ�ϵʽ���ζ����ݣ�����������Ʒ������������
��2��������ͨ�����˷����ʯ�ࣻ����ͼʾ�������Ʒ�к���H+��Ca2+��Mg2+��Fe3+���ӣ�
��3��������ȡ�����õ���������ɣ�������ȡ�����ж���ȡ��Ҫ�������
��4���Ʊ�H3PO4�ij������̿���֪��������ȡ�����⣬���з����⡢ʯ��ȸ���Ʒ�����ݷ������������ʯ�����;��ɣ�
��5��ֱ���������ܽ���ʯ�����ɵ�ʯ����������ʯ���棻
��6���������ɲ���NaH2PO4���������ƵĹ�ϵʽ���ζ����ݣ�����������Ʒ������������
����⣺��1������HF���и�ʴ�ԣ��ܹ���ʴ���������Բ���ʹ�ò������ܽ���ʯ���ʴ�Ϊ�����ܣ�HF�ḯʴ������
��2�����������ù��˲�����ʯ������������ͼʾ���Կ��������������Ʒ�г���H+�⣬������Ca2+��Mg2+��Fe3+�����ӣ�
�ʴ�Ϊ�����ˣ�Ca2+��Mg2+��Fe3+��
��3��������͢�����ȡ�������õ��IJ��������з�Һ©�����ձ�����ȡʱ������������ȡ����ˮ�������ܡ���ȡ��������ˮ�е��ܽ�Ƚ�С�������������Ϲ�ϵ����ad��
�ʴ�Ϊ����Һ©�����ձ��� a��d��
��4�����Ʊ�H3PO4�ij������̿���֪��������ȡ�����⣬���з����⡢ʯ��ȸ���Ʒ����������������̲�����
�ʴ�Ϊ�������⡢ʯ�ࣻ������ܹ���̲�����ֻҪ����������������ʯ�����һ��;���ɣ���
��5�����ֱ���������ܽ���ʯ�����ɵ�ʯ����������ʯ���棬�ù��ձ��������ɵ�ʯ���������ʯ���棬
�ʴ�Ϊ���������ɵ�ʯ���������ʯ���棨�����������𰸣���
��6�������Ʒ�������ǣ�10.00mL��1.526g/cm3=15.26g��1L�����Ʒ��ɵ���Һ����0.103mol/L��NaOH��Һ�����Ϊ��21.35mL��
=1.0675L��
�������������Ʒ�Ӧ�Ĺ�ϵʽΪ��H3PO4��NaH2PO4��NaOH��n��H3PO4��=n��NaOH��=0.103mol/L��1.0675L��0.110mol��
���Ը������Ʒ����������Ϊ��
��100%��70.6%��
�ʴ�Ϊ��70.6%����0.706����
��2�����������ù��˲�����ʯ������������ͼʾ���Կ��������������Ʒ�г���H+�⣬������Ca2+��Mg2+��Fe3+�����ӣ�
�ʴ�Ϊ�����ˣ�Ca2+��Mg2+��Fe3+��
��3��������͢�����ȡ�������õ��IJ��������з�Һ©�����ձ�����ȡʱ������������ȡ����ˮ�������ܡ���ȡ��������ˮ�е��ܽ�Ƚ�С�������������Ϲ�ϵ����ad��
�ʴ�Ϊ����Һ©�����ձ��� a��d��
��4�����Ʊ�H3PO4�ij������̿���֪��������ȡ�����⣬���з����⡢ʯ��ȸ���Ʒ����������������̲�����
�ʴ�Ϊ�������⡢ʯ�ࣻ������ܹ���̲�����ֻҪ����������������ʯ�����һ��;���ɣ���
��5�����ֱ���������ܽ���ʯ�����ɵ�ʯ����������ʯ���棬�ù��ձ��������ɵ�ʯ���������ʯ���棬
�ʴ�Ϊ���������ɵ�ʯ���������ʯ���棨�����������𰸣���
��6�������Ʒ�������ǣ�10.00mL��1.526g/cm3=15.26g��1L�����Ʒ��ɵ���Һ����0.103mol/L��NaOH��Һ�����Ϊ��21.35mL��
| 1000 |
| 20 |
�������������Ʒ�Ӧ�Ĺ�ϵʽΪ��H3PO4��NaH2PO4��NaOH��n��H3PO4��=n��NaOH��=0.103mol/L��1.0675L��0.110mol��
���Ը������Ʒ����������Ϊ��
| 98��0.110 |
| 15.26 |
�ʴ�Ϊ��70.6%����0.706����
����������ͨ����ҵ������ʯΪԭ���Ʊ�H3PO4�ij��÷�������ֿ�����ʵ�������ʵ�����֪ʶ�������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ