��Ŀ����
��11�֣�ijУ��һ��ѧ�о���ѧϰС����ճ������е�������;�����˵��飬�˽�����ɹ㷺��Ӧ���������Ư�ס�ˮ���ɱ���������ȡ�
��1��������������Ư�ס�������ԭ������Ϊ����ˮ�����γɾ���Ư�ס��������õ�_________����ط�Ӧ�����ӷ���ʽΪ____________________________��
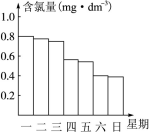
��2)�ڵ���ij������Ӿ���ļ���ˮ�������ʱ��С���Ա�˽������Ӿ��ÿ��һ����8��00��ˮ��Ȼ��ͨ������������ͨ����Ӿ����ˮ�ĺ���������Ч�ȣ�������0.5 mg��L��1��1.0 mg��L��1֮��ʱ��Ч����á���ͼ�Ǹ�С��ⶨ��ÿ��19��00ʱ��Ӿ����ˮ�ĺ��������ļ���ʹ����Ӿ�ز���ȫ��________________��
(3)����Ϊ�ļ�����������ȡ�����ǿ��___________________________��˵��һ��������____________________________________________________(��Ҫ�ķ���ʽ������)��
(4)�ڶ���Ӿ��ˮ��ͨ����������ʱ������������й©ʱ��Ӧ�����ر������ޣ���Ӧ��ȡ�����Ծȷ���______��
(5)С����Ӿ��ͨ��ʹ��Ư��Һ(NaClO��Һ)����������������ˮ���Ծٳ�ʹ��Ư��Һ��������������һ������__________________________________________���û�ѧ����ʽ˵����ҵ���������Ư��Һ��________________________________________________��
��1��������������Ư�ס�������ԭ������Ϊ����ˮ�����γɾ���Ư�ס��������õ�_________����ط�Ӧ�����ӷ���ʽΪ____________________________��
��2)�ڵ���ij������Ӿ���ļ���ˮ�������ʱ��С���Ա�˽������Ӿ��ÿ��һ����8��00��ˮ��Ȼ��ͨ������������ͨ����Ӿ����ˮ�ĺ���������Ч�ȣ�������0.5 mg��L��1��1.0 mg��L��1֮��ʱ��Ч����á���ͼ�Ǹ�С��ⶨ��ÿ��19��00ʱ��Ӿ����ˮ�ĺ��������ļ���ʹ����Ӿ�ز���ȫ��________________��

(3)����Ϊ�ļ�����������ȡ�����ǿ��___________________________��˵��һ��������____________________________________________________(��Ҫ�ķ���ʽ������)��
(4)�ڶ���Ӿ��ˮ��ͨ����������ʱ������������й©ʱ��Ӧ�����ر������ޣ���Ӧ��ȡ�����Ծȷ���______��
| A����ʪ���ë����ס�ڱ�����ʹ� |
| B���ý�ʪС�մ�����ˮ��ë����ס�ڱ�����ߴ� |
| C���ý�ʪŨ��ˮ��ë����ס�ڱ�����������ȫ�� |
| D���ý�ʪʳ��ˮ��ë����ס�ڱ�˳��������ȫ�� |
(��11��)��1���������1�֣�Cl2+H2O�TH++Cl-+HClO��(2��)��2���������������գ���1�֣���3�������ġ�����������1�֣�2HC1O="2HC1+" O2������ǿ��ʱHClO���ֽ⣬�º������½����ԣ�(2��)��4��B��1�֣�
��5��NaClO���ȶ������ڴ�������䣨1�֣�Cl2+2NaOH�TNaCl+ NaClO+ H2O(2��)
��5��NaClO���ȶ������ڴ�������䣨1�֣�Cl2+2NaOH�TNaCl+ NaClO+ H2O(2��)
�����������1����������ˮ���ɴ����ᣬ����������������ɱ����������Ӧ�ķ���ʽ��Cl2+H2O�TH++Cl-+HClO��
��2������ͼ���֪������������������Ӿ����ˮ�ĺ���������0.5mg/L���������������������Dz���ȫ�ġ�
��3�����ڴ������ֽ������Ȼ������������Ӧ�ķ���ʽ��2HC1O="2HC1+" O2��������ǿ��ʱHClO���ֽ⣬�º������½����ԣ����Ը���ͼ���֪��Ӧ���������ġ���������
��4���������ܶȴ��ڿ����ģ�����Ӧ����߳����룬A��C����ȷ��B��ȷ��D����ȷ��Ӧ������糷�룬��ѡB��
��5������NaClO���ȶ������ڴ�������䣬����С����Ӿ��ͨ��ʹ��Ư��Һ����ҵ��ȡƯ��Һ�Ļ�ѧ����ʽ��Cl2+2NaOH�TNaCl+ NaClO+ H2O��
�����������ж��������ڴ�������й¶���¹��С�Ӧ�����ú����������ʣ������������ʺͻ�ѧ���ʡ�������Һ��ѡD����Ϊ���������弫�ӷ������˳�糷�룬������һֱ���ţ�Σ���Ը�������ֻ��������ȷ�ġ���ѧ�ķ������ܽ��ʵ�����⡣

��ϰ��ϵ�д�
�����Ŀ
 SO3
SO3