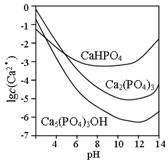
��Ŀ����
��18�֣�������ͼ��ʾ��װ����֤ϡ���ᱻͭ��ԭ�IJ�����NO������NO2��
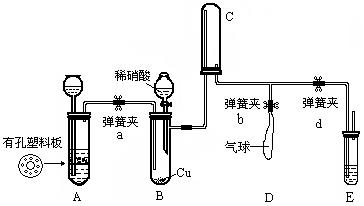
��1��������ʵ��Ӱ��ܴ�Ϊ��Ԥ�ȳ�ȥװ���еĿ�������Aװ���в�����������_________������װ���еĿ����ѱ������ķ�����___________________
_____________________________��E��Ӧ������Լ���_______________��
��2���رյ��ɼ�a��d����b���ɷ�Һ©�����Թ�B�еμ�ϡ���ᣬװ��C�е������__________ɫ��װ��D��������___________________________���رյ��ɼ�d��Ŀ����__________________________________________��
��3����A װ�û��ɹ�����������������C���������ɫ��Ϊ________ɫ����ʱ���ɼ�bӦ��dӦ________������رա�����
��4��ʵ�����ʱ����β�������ʹװ���е��ж����屻E�е���Һ������գ�
__________________________________________________________��

��1��������ʵ��Ӱ��ܴ�Ϊ��Ԥ�ȳ�ȥװ���еĿ�������Aװ���в�����������_________������װ���еĿ����ѱ������ķ�����___________________
_____________________________��E��Ӧ������Լ���_______________��
��2���رյ��ɼ�a��d����b���ɷ�Һ©�����Թ�B�еμ�ϡ���ᣬװ��C�е������__________ɫ��װ��D��������___________________________���رյ��ɼ�d��Ŀ����__________________________________________��
��3����A װ�û��ɹ�����������������C���������ɫ��Ϊ________ɫ����ʱ���ɼ�bӦ��dӦ________������رա�����
��4��ʵ�����ʱ����β�������ʹװ���е��ж����屻E�е���Һ������գ�
__________________________________________________________��
��18�֣���ÿ��2�֣�
��1��H2����E�ij��ڴ������������ȣ�����������Һ
��2���ޡ��������壻��ֹһ�������ݳ���3�����أ��ر�
��4�����������������ٽ����ɼ�d��������������ֱ��װ���е�������ɫ��ȫ��ȥ�����������𰸿ɵ÷֣�
��1��H2����E�ij��ڴ������������ȣ�����������Һ
��2���ޡ��������壻��ֹһ�������ݳ���3�����أ��ر�
��4�����������������ٽ����ɼ�d��������������ֱ��װ���е�������ɫ��ȫ��ȥ�����������𰸿ɵ÷֣�
��

��ϰ��ϵ�д�
 �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
�����Ŀ