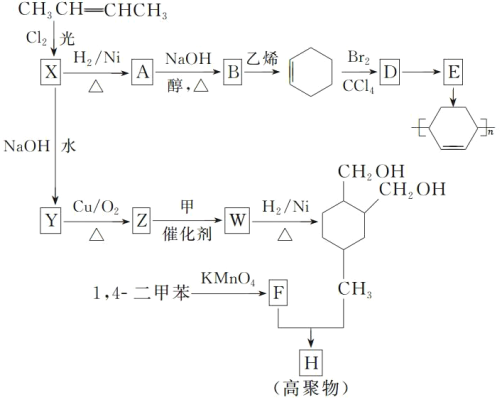
��Ŀ����
����Ŀ������Ҫ�����
(1)CaCO3(s)���ȷֽ�����CaO(s)��CO2(g)����S____________��������>0������<0������=0����
(2)��֪��1 mol H��H���� 1 mol N��H����1 mol N��N���ֱ���Ҫ��������436 kJ��391 kJ��946 kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ___________________________________________________________��
(3)��֪��![]() ��H��-393.5kJ/mol
��H��-393.5kJ/mol
![]() ��H��-571.6kJ/mol
��H��-571.6kJ/mol
![]() ��H��-2599 kJ/mol
��H��-2599 kJ/mol
����298Kʱ��C(s��ʯī)��H2(g)���� 1 mol C2H2(g)��Ӧ���ʱ䣺![]() ___________________��
___________________��
(4)ij�о�����������ͼ��ʾװ���õ绯ѧԭ���������ᡣ

ͨ��SO2��һ���缫��Ӧʽ��__________________________________
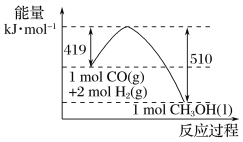
(5)��25 �桢101 kPa�£�1 g�״�ȼ������CO2��Һ̬ˮʱ����22.68 kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ______________________________��
���𰸡�>0 ![]() 226.7 kJ��mol��1
226.7 kJ��mol��1 ![]()
![]()
��������
��1���������ֵ�������壬���̼��ƷֽⷴӦ����������Ӧ������S>0��
��2��N2��H2��Ӧ����NH3�Ļ�ѧ����ʽΪN2��3H2![]() 2NH3���䷴Ӧ������ܺ�=��946+436��3��kJ/mol=2254kJ/mol�����������=391��3��2 kJ/mol=2346kJ/mol���䷴Ӧ�ʱ�
2NH3���䷴Ӧ������ܺ�=��946+436��3��kJ/mol=2254kJ/mol�����������=391��3��2 kJ/mol=2346kJ/mol���䷴Ӧ�ʱ�![]() H=2254kJ/mol-2346kJ/mol=-92 kJ/mol����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ��
H=2254kJ/mol-2346kJ/mol=-92 kJ/mol����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ��![]() ��
��
��3���Ӧ��![]() ��H��-393.5kJ/mol
��H��-393.5kJ/mol
��![]() ��H��-571.6kJ/mol
��H��-571.6kJ/mol
��![]() ��H��-2599 kJ/mol
��H��-2599 kJ/mol
���ݸ�˹���ɣ�������2+����![]() -����
-����![]() �ɵã�2C(s��ʯī)��H2(g)=C2H2(g) ��H��[��-393.5����2+��-571.6����
�ɵã�2C(s��ʯī)��H2(g)=C2H2(g) ��H��[��-393.5����2+��-571.6����![]() -��-2599����
-��-2599����![]() ] kJ/mol=+226.7 kJ/mol��
] kJ/mol=+226.7 kJ/mol��
��4����ͼ��֪��ͨ���SO2����������Ӧ������SO42-����ͨ��SO2��һ���缫��ӦʽΪ��![]() ��
��
��5����25 �桢101 kPa�£�1 g�״�ȼ������CO2��Һ̬ˮʱ����22.68 kJ����1mol�״���ȫȼ�����ų�������Ϊ32��22.68 kJ=725.76kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��![]() ��
��

 ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д�