��Ŀ����
����Ŀ����һ������������������������������·�Ӧ��2SO2(g)��O2(g)![]() 2SO3(g) ��H ��0
2SO3(g) ��H ��0
��1�������¶ȣ��÷�ӦKֵ______����������ת����______��(���Ͼ���������������С������������)
��2��600 ��ʱ����һ�ܱ������У������������������ϣ���Ӧ������SO2��O2��SO3���ʵ����仯��ͼ��ʾ����Ӧ����ƽ��״̬��ʱ����______________________��
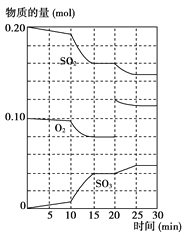
��3������ͼ�жϣ���Ӧ������20 minʱ�����߷����仯��ԭ����__________________(�����ֱ���)��
��4������������Ӧ��ij����������˵���÷�Ӧ�Ѵﵽƽ��״̬����_______��
A .SO2������������SO3�������������
B. ����a mol SO2��ͬʱ����a mol SO3
C. �����Ũ�Ȳ��ڷ����仯
D.������ϵ��ѹǿ���ٷ����仯
E. ������ϵ�������ܶȲ��ٱ仯
F. ��H���ٷ����仯
G.������SO2��O2��SO3��Ũ�ȱ���2:1:2
���𰸡� ���� ���� 15��20 min��25��30 min ������O2���� A��C��D
��������(1)2SO2(g)+O2(g)2SO3(g)��H��0����Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ��������У�ƽ�ⳣ������������ת�������ʴ�Ϊ����������
(2)��Ӧ���������ʵ����ʵ������仯��˵����Ӧ����ƽ��״̬����ͼ��֪��15-20min��25-30min����ƽ̨������ֵ����ʵ������䣬��Ӧ����ƽ��״̬���ʴ�Ϊ��15-20min��25-30min��
(3)��ͼ��֪����Ӧ������20minʱ��ƽ��������Ӧ�ƶ���˲��ֻ��������Ũ������Ӧ��������������Ũ�ȣ��ʴ�Ϊ������������Ũ�ȣ�
(4) A .SO2������������SO3������������ȣ���ʾ���淴Ӧ������ȣ�˵���÷�Ӧ�Ѵﵽƽ��״̬����ȷ��B. ����a mol SO2��ͬʱ�ض�����a mol SO3������˵���÷�Ӧ�Ѵﵽƽ��״̬��������C. �����Ũ�Ȳ��ڷ����仯����ʾ�÷�Ӧ�Ѵﵽƽ��״̬����ȷ��D.�÷�Ӧ������������ʵ�����С�ķ�Ӧ��������ϵ��ѹǿ���ٷ����仯��˵�����ʵ������ٱ仯��˵���÷�Ӧ�Ѵﵽƽ��״̬����ȷ��E. ������ϵ���������������������䣬�ܶ�ʼ�ղ��䣬����˵���÷�Ӧ�Ѵﵽƽ��״̬������F. ��H�뷴Ӧ���еij̶��أ�����˵���÷�Ӧ�Ѵﵽƽ��״̬������ G.ƽ��ʱ������SO2��O2��SO3��Ũ�ȱȲ�һ����2:1:2������˵���÷�Ӧ�Ѵﵽƽ��״̬������ѡACD��

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�