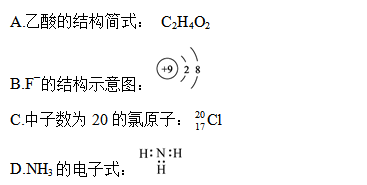
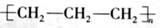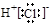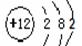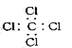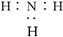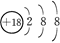��Ŀ����
�ƽ�����ʢ��ǿ��ԭ���£�N2H4����ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų������ȡ���֪0.4molҺ̬��������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256.652KJ��������
��1��д����������ĵ���ʽ ��
��2���÷�Ӧ���Ȼ�ѧ����ʽΪ ��
1mol����ȫ��Ӧת�Ƶ����� ��
��3���˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��� ��
��4������֪H2O(l)==H2O(g)����H = +44kJ?mol-1����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų��������� kJ��
��1��д����������ĵ���ʽ ��
��2���÷�Ӧ���Ȼ�ѧ����ʽΪ ��
1mol����ȫ��Ӧת�Ƶ����� ��
��3���˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��� ��
��4������֪H2O(l)==H2O(g)����H = +44kJ?mol-1����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų��������� kJ��
��1�� ��2��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g�� ��H=-641.6KJ/mol��4NA
��2��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g�� ��H=-641.6KJ/mol��4NA
��3������������Ⱦ ��4��408.8
 ��2��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g�� ��H=-641.6KJ/mol��4NA
��2��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g�� ��H=-641.6KJ/mol��4NA��3������������Ⱦ ��4��408.8
�����������1�����������������ԭ������ԭ��֮���γɼ��Լ�����ԭ������ԭ��֮���γɷǼ��Լ�������ʽΪ
 ��
����2����֪0.4molҺ̬�º�����˫��ˮ��Ӧ���ɵ�����ˮ����ʱ�ų�256.652KJ���������º�˫��ˮ��Ӧ���Ȼ�ѧ����ʽ��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g�� ��H=-641.6KJ/mol���·����е�Ԫ�صĻ��ϼ��ǣ�2�ۣ���Ӧ���Ϊ0�ۣ�ʧȥ2�����ӣ���1mol����ȫ��Ӧת�Ƶ�����Ϊ4NA��
��3����ԭ���£�N2H4����ǿ������H2O2�������ǻ��ʱ�������������ĵ�����ˮ���������ͷŴ��������Ϳ��ٲ������������⣬���к�ͻ�����ŵ��ǣ�����Ϊ������ˮ������Ⱦ���ʴ�Ϊ������Ϊ������ˮ������Ⱦ��
��4����N2H4��l��+2H2O2��l���TN2��g��+4H2O��g������H=-641.6KJ/mol����H2O��l��=H2O��g������H=+44KJ/mol�����ݸ�˹���ɢ�-�ڡ�4�õ���N2H4��l��+2H2O2��l���TN2��g��+4H2O��L������H=-817.6KJ/mol����ѧ����ʽ��32gȫ����Ӧ����817.6KJ��16gҺ̬��������˫��ˮ��Ӧ���ɵ�����Һ̬ˮʱ���ų���������408.8KJ��

��ϰ��ϵ�д�
�����Ŀ