��Ŀ����
CoCl2•xH2O��Na2CO3•10H2O�Ĺ������ﹲ8.100g������������ˮ�����ɰ�ɫ��̼���ܳ������������˳���ϴ����ɣ�������Ϊ1.190g������Һ��ϴ��Һ�ϲ��������������������ɫ����0.224L(������������ܽ�)��
��1������������Na2CO3•10H2O������Ϊ_______ g������2λС������
��2��CoCl2•xH2O ��x=_____��
��3�����õ���1.190g�����ڿ����г�����������أ��õ��Ĺ�����һ���ܵ������������Ϊ0.830g���������������Ӿ��塣ͨ�����㣬��ȷ���þ���Ļ�ѧʽΪ ��
����6�֣���2�֣�
��1��5.72 ��2��6 ��3��Co2O3

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д��о����ʵ��۽ṹ�������������������ʱ仯�ı��ʡ���ش�
��1��C��Si��NԪ�صĵ縺���ɴ�С��˳���� ��C60�ͽ��ʯ����̼��ͬ�������壬���ʯ�۵����C60���۵㣬ԭ���� ��
��2�� A��B��Ϊ�����ڽ���Ԫ�ء������±����ݣ�д��Bԭ�ӵĵ����Ų�ʽ ��
|
������/kJ•mol��1 |
I1 |
I2 |
I3 |
I4 |
|
A |
932 |
1821 |
15390 |
21771 |
|
B |
738 |
1451 |
7733 |
10540 |
��3�����ɽ���������ˮ�����γɵ�������Ƿ�����ɫ������d��������Ų��йء�һ��أ�Ϊd0��d10�Ų�ʱ������ɫ��Ϊd1��d9�Ų�ʱ������ɫ����[Co(H2O)6]2+�Էۺ�ɫ���ݴ��жϣ�[Mn(H2O)6]2+ ��ɫ����ޡ����С�����
��4������CO���Ժϳɻ���ԭ��COCl2�������Fe(CO)5�ȡ�
�� COCl2���ӵĽṹʽΪ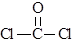 ��COCl2�����ں���
�����ţ���
��COCl2�����ں���
�����ţ���
A��4���Ҽ� B��2���Ҽ���2���м� C��2���Ҽ���1���м� D��3 ���Ҽ���1���м�
�� Fe(CO)5��һ�������·����ֽⷴӦ��Fe(CO)5��Fe(s)��5CO����Ӧ�����У����ѵĻ�ѧ��ֻ����λ�����γɵĻ�ѧ���� ��


