��Ŀ����
������ˮ��Һ�п��ܴ��ڵ���ƽ�⡢�ε�ˮ��ƽ�������ܽ�ƽ�⣬���Ƕ��ɿ�����ѧƽ���һ�֡��������ѧ��ѧ֪ʶ�ش��������⣺
��1����0��1 mol��L-1��(NH4)2SO4��Һ���ڸ���Һ������Ũ���ɴ�С��˳��Ϊ________________��
��2����0��1 mol��L-1��NaHCO3��Һ���ڸ���Һ�д��ڵ�ƽ������������ֱ������ӷ���ʽ��ʾ��
____________________________________________________________��
��3����֪(NH4)2Fe(SO4)2����ˮȫ�������NH4+��Fe2+��SO42-������ͬŨ�ȵ�(NH4)2Fe(SO4)2��
(NH4)2SO4��Һ����__________________��Һ��NH4+Ũ�ȸ����ѧʽ������ԭ����______________________________��
��4��AgCl����Һ�д�������ƽ�⣺AgCl(s)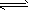 Ag+(aq)��Cl-(aq)����25�� ʱ��AgCl��Ksp��1��8��10-10���ֽ�����AgCl�ֱ��������Һ���У��� 100mL����ˮ���� 100mL 0��3mol��L-1 AgNO3��Һ���� 100mL
Ag+(aq)��Cl-(aq)����25�� ʱ��AgCl��Ksp��1��8��10-10���ֽ�����AgCl�ֱ��������Һ���У��� 100mL����ˮ���� 100mL 0��3mol��L-1 AgNO3��Һ���� 100mL
0��1 mol��L-1MgCl2��Һ��ֽ������ȴ����ͬ�¶ȣ�Ag+Ũ���ɴ�С��˳��Ϊ________________(�����)��
��1��c(NH4+)>c(SO42-)>c(H+)>c(OH-)
��2��HCO3-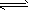 H++CO32-��HCO3-+H2O
H++CO32-��HCO3-+H2O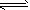 H2CO3+OH-��H2O
H2CO3+OH-��H2O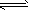 H++OH-
H++OH-
��3��(NH4)2Fe(SO4)2��Fe2+ˮ�������ԣ���NH4+��ˮ������������
��4���ڢ٢�
��2��HCO3-
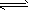 H++CO32-��HCO3-+H2O
H++CO32-��HCO3-+H2O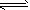 H2CO3+OH-��H2O
H2CO3+OH-��H2O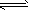 H++OH-
H++OH-��3��(NH4)2Fe(SO4)2��Fe2+ˮ�������ԣ���NH4+��ˮ������������
��4���ڢ٢�

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ