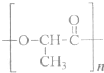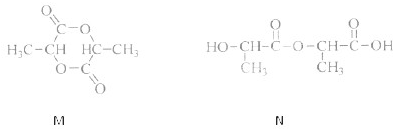��Ŀ����
��������ɸ�������ɺ����ʽ��з��ࣺ
��1��������ʾ�����ʷ����������
��2����Na��K��H��O��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��Тڢۢ��森��������дһ����ѧʽ���ɣ�
��3��д����ת��Ϊ�ݵĻ�ѧ����ʽ
��4��д������۷�Ӧ�����ӷ���ʽ
��5��д������ݷ�Ӧ�����ӷ���ʽ
��6��д������Zn��Ӧ�Ļ�ѧ����ʽ����˫���ŷ��������ת�Ʒ������Ŀ��
 ����������
����������

��1��������ʾ�����ʷ����������
��״���෨
��״���෨
����2����Na��K��H��O��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��Тڢۢ��森��������дһ����ѧʽ���ɣ�
| ������� | �� | �� | �� | ������ | �⻯�� |
| ��ѧʽ | ��HCl �� H2SO4 H2SO4 |
�� NaOH NaOH ��Ba��OH��2 |
��Na2CO3 �� K2SO4 K2SO4 |
��CO2 ��Na2O |
��NH3 ��H2O |
CO2+2NaOH�TNa2CO3+H2O
CO2+2NaOH�TNa2CO3+H2O
��4��д������۷�Ӧ�����ӷ���ʽ
H++OH-�TH2O
H++OH-�TH2O
��5��д������ݷ�Ӧ�����ӷ���ʽ
2H++CO32-�TCO2��+H2O
2H++CO32-�TCO2��+H2O
��6��д������Zn��Ӧ�Ļ�ѧ����ʽ����˫���ŷ��������ת�Ʒ������Ŀ��


HCl
HCl
��ԭ����Zn
Zn
����������1��������״���෨���������
��2����������Ρ������P�⻯��ĺ��������Ԫ�ط�����
��3��CO2��NaOH��Ӧת��Ϊa2CO3��
��4��HCl�����кͷ�Ӧ����ˮ��
��5��ǿ����Na2CO3��Ӧ���ɶ�����̼��ˮ��
��6��HCl��Zn��Ӧ�����Ȼ�п�����������ݻ��ϼ۵ı仯��������ת�ƣ����������ͻ�ԭ����
��2����������Ρ������P�⻯��ĺ��������Ԫ�ط�����
��3��CO2��NaOH��Ӧת��Ϊa2CO3��
��4��HCl�����кͷ�Ӧ����ˮ��
��5��ǿ����Na2CO3��Ӧ���ɶ�����̼��ˮ��
��6��HCl��Zn��Ӧ�����Ȼ�п�����������ݻ��ϼ۵ı仯��������ת�ƣ����������ͻ�ԭ����
����⣺��1����״���෨��һ�ֺ�����ķ��෨�����ղ�Σ�һ��һ�����֣�����һ�ô�������Ҷ��֦���ˡ�����ͼʾ����������״ͼ���ʴ�Ϊ����״���෨��
��2����Na��K��H��O��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ���������������ȫ���������ӵĻ������H2SO4����������������ȫ��Ϊ���������ӣ���NaOH���ε������������Ϊ�������ӻ�笠����ӣ�������Ϊ������ӣ���K2SO4������
�ʴ�Ϊ��
��3��CO2��NaOH��Ӧת��Ϊa2CO3���仯ѧ����ʽΪ��CO2+2NaOH�TNa2CO3+H2O���ʴ�Ϊ��CO2+2NaOH�TNa2CO3+H2O��
��4��HCl�����кͷ�Ӧ�����κ�ˮ�������ӷ���ʽΪH++OH-�TH2O���ʴ�Ϊ��H++OH-�TH2O��
��5��ǿ����Na2CO3��Ӧ���ɶ�����̼��ˮ�������ӷ���ʽΪ��2H++CO32-�TCO2��+H2O���ʴ�Ϊ��2H++CO32-�TCO2��+H2O��
��6��HCl��Zn��Ӧ�����Ȼ�п��������Zn���ϼ�����2��ʧȥ2�����ӣ�����ԭ����HCl���ϼ۽�������������˫���ŷ���ʾ��Ӧ����Ϊ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� �� HCl�� Zn��
�� HCl�� Zn��
��2����Na��K��H��O��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ���������������ȫ���������ӵĻ������H2SO4����������������ȫ��Ϊ���������ӣ���NaOH���ε������������Ϊ�������ӻ�笠����ӣ�������Ϊ������ӣ���K2SO4������
| ������� | �� | �� | �� | ������ | �⻯�� |
| ��ѧʽ | ��HCl ��H2SO4 |
��NaOH ��Ba��OH��2 |
��Na2CO3 ��K2SO4 |
��CO2 ��Na2O |
��NH3 ��H2O |
| ������� | �� | �� | �� | ������ | �⻯�� |
| ��ѧʽ | ��HCl ��H2SO4 |
��NaOH ��Ba��OH��2 |
��Na2CO3 ��K2SO4 |
��CO2 ��Na2O |
��NH3 ��H2O |
��4��HCl�����кͷ�Ӧ�����κ�ˮ�������ӷ���ʽΪH++OH-�TH2O���ʴ�Ϊ��H++OH-�TH2O��
��5��ǿ����Na2CO3��Ӧ���ɶ�����̼��ˮ�������ӷ���ʽΪ��2H++CO32-�TCO2��+H2O���ʴ�Ϊ��2H++CO32-�TCO2��+H2O��
��6��HCl��Zn��Ӧ�����Ȼ�п��������Zn���ϼ�����2��ʧȥ2�����ӣ�����ԭ����HCl���ϼ۽�������������˫���ŷ���ʾ��Ӧ����Ϊ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� �� HCl�� Zn��
�� HCl�� Zn�����������⿼�����ʵķ��࣬���ӷ���ʽ����д��������ԭ��Ӧ����Ŀ�ѶȲ���ע�������ظ��

��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ