��Ŀ����
��һ�����ʵ���Ũ����Һ�����ƺ�����к͵ζ�����ѧ��ѧ���������͵Ķ���ʵ�顣ij�о���ѧϰС����ʵ����������1mol/L��ϡ�������Һ��Ȼ������ζ�ijδ֪Ũ�ȵ�NaOH��Һ�������й�˵������ȷ����______________���𰸿��ܲ�Ψһ��
A��ʵ�������õ��ĵζ��ܡ�����ƿ����ʹ��ǰ����Ҫ��©��
B�����ʵ��������60mL ��ϡ�������Һ������ʱӦѡ��100mL����ƿ��
C������ƿ�к�����������ˮ���ᵼ���������Һ��Ũ��ƫС��
D����ʽ�ζ���������ˮϴ�Ӻ�װ���Ũ�ȵ�ϡ���ᣬ���õ�NaOH��Һ��Ũ�Ƚ�ƫ��
E���ü�����ָʾ�����ζ��յ�ʱ����Һ��ɫ�ӳ�ɫ��Ϊ��ɫ��
F��������Һ���к͵ζ�������ʵ���У��������һ�ζ��������Ӷ���������ʵ������ƫ��
�� . ������ͼ��ʾ��װ����ȡ�϶����ı�����ˮ���ⶨ������ˮ��pH��
�ش��й����⣺
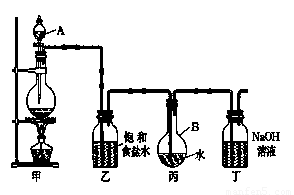
��1��д���йػ�ѧ����ʽ��
װ�üף�____________________________________ ��
װ�ö��� __________________ ��
��2��֤����ˮ�ѱ��͵������� ��
��3����ȡ����ʱ��װ�ñ���Һ���к��е����� ���������ű�ʾ ����
(4)����ȥװ���ң�ֱ�ӽ�װ�üͱ���������������ʵ��ⶨ�����Ӱ���ǣ� ��
�ⶨ������ˮ��pH������_______________________________________________��
��.ʵ����ƣ�֤��NaOH�����ڿ����з��ò��ֱ���
_______________________________________________________________
_______________________________________________________________________
�� �� ��16�֣� ��3�֣� A��B��D�� (��ѡ���÷֣���ѡ1���1�� )
��. ��8�֣�����1���ף�MnO2+4HCl MnCl2+Cl2��+2H2O��1�֣�
MnCl2+Cl2��+2H2O��1�֣�
����Cl2+2NaOH��NaCl+NaClO+H2O��1�֣�
��2������Һ��ʻ���ɫ�����ϲ��ռ�ʻ���ɫ���л���ɫ������붡�У�2�֣�
��3�� H2O��Na+��Cl-��H+��Cl2 ��HClO��OH-��2�֣���ѡȫ��2�֣�©ѡ��1�֣���ѡ���÷֣�
��4�������Ȼ���δ�����ᵼ���Ƶõ���ˮ������ǿ����õ�pHƫС��1�֣���
��pH��ֱ�Ӳⶨ����pH��ֽ�ⶨ�����֣���1�֣���
��3�֣���ȥ������Ʒ��������ˮ��1�֣�������Һ�м������CaCl2��Һ����Һ������ɫ����˵������Ʒ�ѱ��ʣ�1�֣�����ʱ����Һ�е���2�η�̪������Һ�ʺ�ɫ˵�����в�����������û���ʣ�1�֣�����������Ʒ���ʡ�
����������

 ��һ��������þ���Ͻ�Ͷ��100mlһ�����ʵ���Ũ�ȵ������У��Ͻ�ȫ���ܽ⣬��������Һ�еμ�5mol?L-1 NaOH��Һ�����������ɳ���������������NaOH��Һ�������ϵ��ͼ��ʾ����
��һ��������þ���Ͻ�Ͷ��100mlһ�����ʵ���Ũ�ȵ������У��Ͻ�ȫ���ܽ⣬��������Һ�еμ�5mol?L-1 NaOH��Һ�����������ɳ���������������NaOH��Һ�������ϵ��ͼ��ʾ����