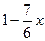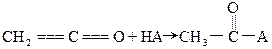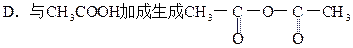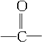��Ŀ����
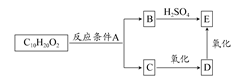
��8�֣��Ĵ�ʢ���屶�ӡ����屶��Ϊԭ�Ͽ��Ƶû�����A��A�Ľṹ��ʽ����ͼ��ʾ��
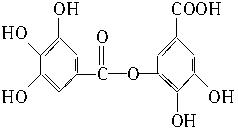
��������и��⣺
��1��A�ķ���ʽ��______________________________��
��2���л�������B������������¼��ȷ���������Ӧ�ɵõ�A����д��B�Ľṹ��ʽ��___________________________________________________________________________��
��3����д��A�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��______________________________��
��4���л�������C�Ǻϳ�����������ҩ���ԭ��֮һ��C���Կ�����B�����������ʵ���֮��1��2�����ӳɷ�Ӧ�õ��IJ��C���������ǻ���̼̼˫��ֱ�������Ľṹ����������ˮ��Ӧʹ��ˮ��ɫ����д��C����ˮ��Ӧ�Ļ�ѧ����ʽ��

��������и��⣺
��1��A�ķ���ʽ��______________________________��
��2���л�������B������������¼��ȷ���������Ӧ�ɵõ�A����д��B�Ľṹ��ʽ��___________________________________________________________________________��
��3����д��A�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��______________________________��
��4���л�������C�Ǻϳ�����������ҩ���ԭ��֮һ��C���Կ�����B�����������ʵ���֮��1��2�����ӳɷ�Ӧ�õ��IJ��C���������ǻ���̼̼˫��ֱ�������Ľṹ����������ˮ��Ӧʹ��ˮ��ɫ����д��C����ˮ��Ӧ�Ļ�ѧ����ʽ��
��1��C14H10O9 ��2��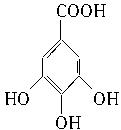
��3��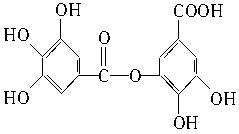 +8NaOH��2
+8NaOH��2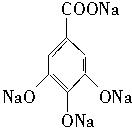 +7H2O
+7H2O
��4��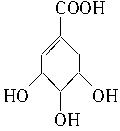 +Br2��
+Br2��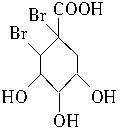

��3��
 +8NaOH��2
+8NaOH��2 +7H2O
+7H2O��4��
 +Br2��
+Br2��
��2�������⣺2����B����������Ӧ��1����A���ʿɸ���A�Ľṹ��ʽ�Ƴ�B�Ľṹ��ʽΪ��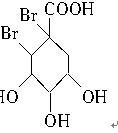 ��
��
��3��A�����NaOH������Ӧ�����ǻ�������������NaOH��Ӧ����1mol A����8mol NaOH��
��4�����������Ϣ�ƶϣ�CΪ ������Br2�����ӳɷ�Ӧ��
������Br2�����ӳɷ�Ӧ��
 ��
����3��A�����NaOH������Ӧ�����ǻ�������������NaOH��Ӧ����1mol A����8mol NaOH��
��4�����������Ϣ�ƶϣ�CΪ
 ������Br2�����ӳɷ�Ӧ��
������Br2�����ӳɷ�Ӧ��
��ϰ��ϵ�д�
�����Ŀ